Translate this page into:
Molecular Structure, Biochemical Functions, Genetics, and Emerging Clinical Relevance of Glucose Transporters

-
Received: ,
Accepted: ,
How to cite this article: Jafri SQ, Shah SIA, Jaffari SHA. Molecular Structure, Biochemical Functions, Genetics, and Emerging Clinical Relevance of Glucose Transporters. Glob J Med Pharm Biomed Update 2023;18:23.
Abstract
In the human body, glucose acts as a major energy-producing fuel and regulator of energy homeostasis, enzyme functions, and gene transcription. The selective permeability of the lipid bilayer structure of the cell membrane makes it mandatory for glucose to require transport proteins for its transit into the cells. These include solute carrier integral membrane proteins such as glucose transporters (GLUTs) and sodium-glucose transporters. GLUTs belong to the major facilitator superfamily with a 12 transmembrane spanner topology, with GLUT1–13 sharing the same transmembrane sequence but variable transmembrane loops and terminal cytoplasmic ends of carbon and nitrogen. Phylogenetic analysis classifies GLUTs into three classes, with each class showing an affinity for a specific substrate. The tightly coupled relationship between glucose homeostasis and the nearly ubiquitous GLUTs has led to the investigation of their diverse roles in embryonic development, adult physiology, and clinical disorders including but not limited to inborn errors, diabetes mellitus, metabolic syndrome, and cancers. The current review is pivoted around the studies focusing on the structure and functions of members of the GLUT family, their chromosomal and organ-specific distribution, as well as the current evidence of their clinical implications and prospective therapeutic roles, specifically in cancers and metabolic disorders. The literature for the present work was retrieved from databases including Google Scholar, Web of Science, and PubMed.
Keywords
Glucose
Glucose transporters
Energy
Glucose metabolism
Glucose homeostasis
INTRODUCTION
Glucose serves as a major source of energy for most of the mammalian cells. It is produced as a result of carbon assimilation by the photosynthetic organisms such asplants, Algae, bacteria, and Euglena.It plays a major role in various catabolic and anabolic chemical reactions carried out in the human body. It also acts as a substrate for the synthesis of other biochemical molecules and as a cell signaling molecule to maintain energy homeostasis. Glucose also acts as a major regulator of gene transcription, enzyme activity, hormone secretion, and glucoregulatory neurons. Humans consume carbohydrates containing glucose in monomers, dimers, oligomers, and polymers such as sucrose, lactose, starch, and cellulose. In humans, the brain constitutes only ~2% of total body weight but uses ~20% of energy derived from glucose; thus, it is the major utilizer of glucose (~5.6 mg glucose/100 g human brain tissue/min).[1]
Glucose itself is strongly hydrophilic, but given the nature of the phospholipid bilayer, it requires certain solute carrier (SLC) transport proteins to facilitate its transport along or against a concentration gradient without the expenditure of any adenosine triphosphate (ATP). SLC membrane transport proteins are integral proteins present on the cell surface and organelle membranes and include facilitative transporters and secondary active transporters.[2]
MAIN TYPES OF TRANSPORTERS
In the human genome, three families of SLC membrane transport proteins have been identified: SLC2, SLC5, and SLC50.[3] SLC2. They are commonly known as glucose transporters (GLUTs), and consist of three classes.They have a ubiquitous distribution found in almost all the cells of the body to facilitate the transport of glucose into the cell through facilitated diffusion. SLC5 or sodium-glucose transporters (SGLTs) found predominantly in the apical membrane of intestinal epithelium (SGLT1) and renal tubular cells (SGLT2) work by actively cotransporting glucose against its concentration gradient using sodium motive force generated by the electrochemical gradient of sodium.[4,5] SLC50 or Sugars Will Eventually be Exported Transporters (SWEET) are newly discovered transporters found widely in plants and play a physiological role in facilitating the transport of sugars across cell membranes along a concentration gradient.[6]
GLUTs
The family includes facilitative GLUTs that typically carry various hydrophilic hexose molecules across the hydrophobic cell membranes in a tissue and substrate-specific manner, along the concentration gradient. GLUTs are categorized into three subfamilies based on sequence similarities, characteristic elements, and functional properties.[7]
Structure
A detailed search of the GenBank database showed that all GLUTs involved in the facilitated diffusion of glucose are distributed in various tissues in humans.[8] They belong to the major facilitator superfamily (MFS) and display the distinct tertiary structure of the MFS 12 transmembrane spanner (TMS) topology.[9] A comparison of sequences among all 13 members elucidated that the sequence was preserved in the TM region whereas showed more variation in the TM loops and the terminal ends of carbon and nitrogen present on the cytoplasmic side of the plasma membrane.[10]
Among various GLUTs that have been identified, GLUT1 was the first GLUT whose isomer was isolated and cloned from bacterial homolog[11] of D-Xylose: H+ known as XylE symporter. This was obtained from Escherichia coli sharing 29% sequence identity and 49% sequence similarity with GLUT1.[12]
Based on similarities in the sequence obtained from phylogenetic analysis,[13] GLUTs have been classified into three classes [Figure 1].[14]
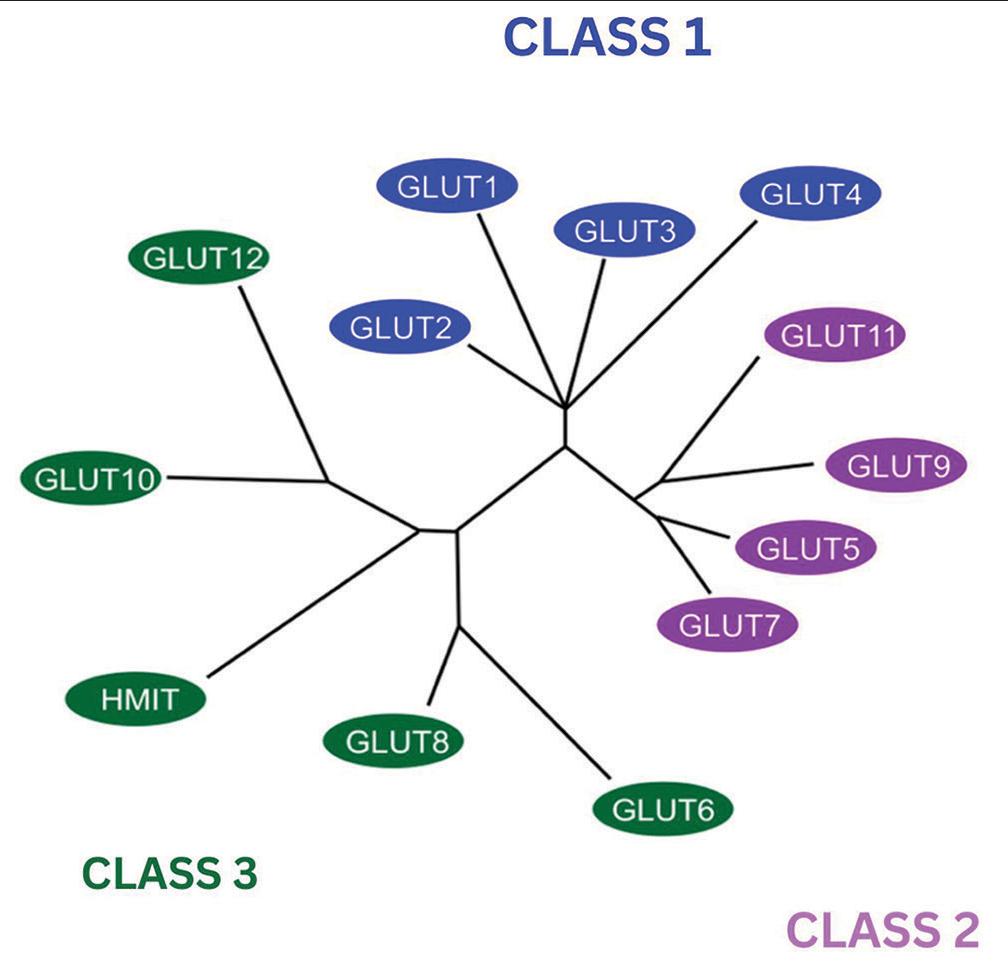
- Phylogenetic dendrogram of glucose transporters (GLUTs) on the basis of similarities in their sequence modified from the image initially published by Wood and Trayhurn in GLUTs (GLUT and sodium-glucose transporters): Expanded families of sugar transport proteins in the British Journal of Nutrition. The blue colored boxes depict GLUT Class 1, the Purple color shows Class 2 and Green color shows Class 3.
Class 1 (GLUT1–4)
The structure of the isoforms of class 1 GLUTs has been identified using the hydropathy model, an analyzing technique wherein the structure of proteins is studied based on its interaction with the water molecules.[15] This model depicted that all the isoforms share the same 12 TMS polypeptide backbone with variations in −NH2 and −COOH side chains, making the isoforms distinct from one another. The first two transmembrane segments are connected by an extracellular loop of 33, 37, and 67 amino acids for GLUT1 and 3, GLUT4, and GLUT2, respectively. The sixth and seventh segments are connected by an intracellular loop comprising 65 amino acids on the cytoplasmic side of the lipid bilayer. The sequence of these transmembrane segments and the connecting intracellular loops are the most conserved regions in all the isoforms of this class of GLUT and, hence, may promote a common function of facilitative glucose transportation.[16] The less conserved regions of −NH2 and − COOH might be attributed to specific characteristics of each isoform such as hormonal responsivity and upregulation in response to specific stimulus.[17] Glutamine and Tryptophan in transmembrane 5 and STSIF motif in loop 7 are two of the few classes of specific residue. A quasielastic light scattering (QLS) motif depicting glucose specificity in TM 7 is also a distinct feature of GLUT1, 3, and 4.[18]
Class 2 (GLUT 5, 7, 9, and 11)
Class 2 has more substrate specificity for fructose and to a lesser extent for glucose. This class is deprived of the tryptophan residue post-GPXXXP motif on TM10 with the absence of the QLS motif as well.[19] A major feature that distinguishes class 1 from class 2 is that the latter has a solitary hydrophobic isoleucine residue on TM 7 providing substrate specificity for fructose, except for GLUT11 which has a valine instead of isoleucine but at a different primary sequence. This property allows it to transport both glucose and fructose.[20] Genome-wide association studies have also identified GLUT9 as a high-capacity urate transporter.[21]
Class 3 (GLUT 6, 8, 10, 12, and 13)
This class of facilitative transporters consists of GLUT 6, 8, 10, 12, and 13, also known as H+-myo-inositol transporter (HMIT), ascribed to its proton-coupled function. The main characteristic feature of this class is the presence of a glycosylation site on loop 9 (between TM 9 and 10) instead of loop 1 (between TM 1 and 2) as is found in classes 1 and 2 of GLUTs.[14] Like class 2, this class also lacks a QLS motif but many other motifs are conserved that include the likes of PESPR in TM6 and PETKGR in TM12. Like Class 1, it also has a tryptophan residue after the GPXXXP motif in TM10.[19]
GLUT13 (or HMIT) is the only GLUT with highly electrogenic substrate specificity for myo-inositol, a substrate required for adequate brain functioning. The coupling of GLUT13 with proton is responsible for its stimulation with low pH. No glucose transport activity has yet been associated with GLUT13 [Figure 2].[22]
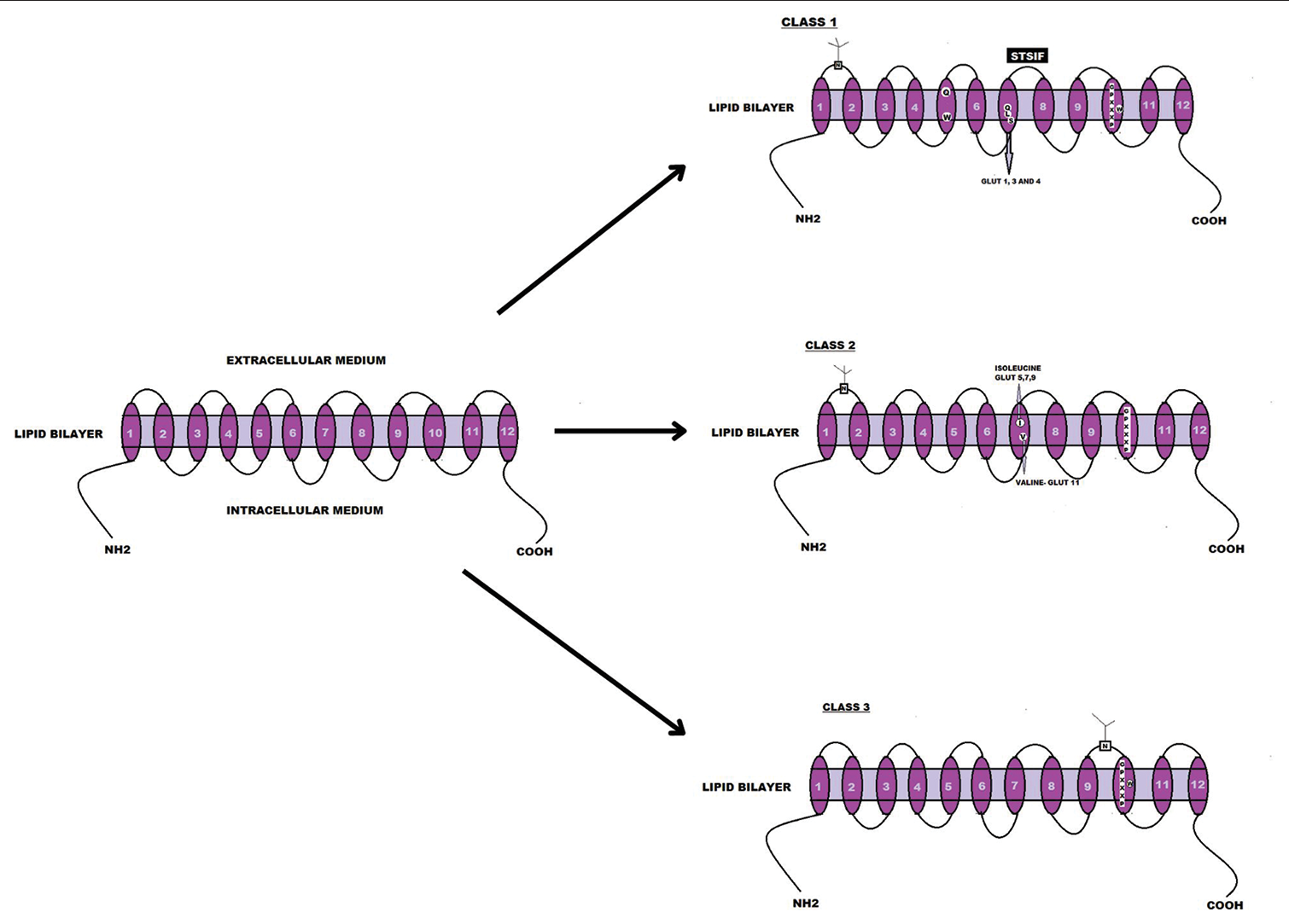
- Classification and pictorial comparison of the three classes of glucose transporters on the basis of their structure and the presence of TM motifs. The image was designed using Canva.
Genetics
The search conducted using a basic local alignment search tool on GenBank human genome database[10] revealed that all the genes coding for GLUT consists of only a single copy with the exception of GLUTs 9 and 11, which show at least three variants mainly as a result of splicing.[23] These genes are distributed over the range of 9 chromosomes in the human body. GLUT1, 5, and 7 are found on chromosome 1. GLUT 6 and 8 are found closely associated with one another on chromosome 9. GLUT 3 and HMIT are present on chromosome 12. GLUT 2, 4, 9, 10, 11, and 12 are each located on chromosomes 3, 17, 4, 20, 22, and 6, respectively [Table 1]. With the exception of GLUT9 (214 kb) and HMIT (352 kb), the sizes of gene-encoding gluts are relatively small ranging from 6 kb to 65 kb.[10]
| GLUTs | Gene | Chromosomal location |
|---|---|---|
| GLUT1 | SLC2A1 | 1p35-p31.3 |
| GLUT2 | SLC2A2 | 3q26-q26.2 |
| GLUT3 | SLC2A3 | 12p13.3 |
| GLUT4 | SLC2A4 | 17p13 |
| GLUT5 | SLC2A5 | 1p36.2 |
| GLUT6 | SLC2A6 | 9q34 |
| GLUT7 | SLC2A7 | 1p36.22 |
| GLUT8 | SLC2A8 | 9q33.3 |
| GLUT9 | SLC2A9 | 4p16-p15.3 |
| GLUT10 | SLC2A10 | 20q13.1 |
| GLUT11 | SLC2A11 | 22q11.2 |
| GLUT12 | SLC2A12 | 6q23.2 |
| GLUT13 (HMIT) | SLC2A13 | 12q12 |
GLUTS: Glucose transporters, SLC: Solute carrier, HMIT: H+-myo-inositol transporter
TYPES OF GLUTs
Human GLUT1 was obtained on sodium-dodecyl-sulfate polyacrylamide gel from human erythrocytes in the year 1977 by Kasahara and Hinkle.[10,24] GLUT1 is a hydrophobic integral membrane comprising 492 amino acids with a molecular weight of 54 kDa. Also known as HepG2, GLUT1 has a vital function in transporting glucose as well as galactose, mannose, glucosamine, and ascorbic acid. Structurally, it consists of 12 hydrophobic transmembrane α-helices with glycosylated extracellular loops positioned between the first and second transmembrane helices. GLUT1 expression is most prominent in proliferating cells during early embryo development and is concentrated in the brain, skeletal muscle, and myocardium during the suckling phase. Subsequently, its expression decreases in most tissues except for the brain, where it remains consistent. GLUT1 is widespread in cells forming blood-tissue barriers, including those in the brain, retina, nerves, and endothelium. In addition, it is found in cardiac muscle, placenta, and lactating mammary glands. In insulin-sensitive tissues such as adipose tissue and muscle, GLUT1 is associated with GLUT4.[7]
GLUT1 present in endometrial stromal cells of the placenta and lactating mammary glands plays a key role in the maintenance of a healthy pregnancy by providing adequate energy to the developing fetus.[25] Glucose is essentially required for embryonic development and endometrial decidualization and abnormal endometrial glucose metabolism is linked with negative gestational outcomes. Glucose cannot be produced in the endometrium; hence, it is delivered by GLUTs. Expression of seven of the GLUTs has been observed in the human uterus which is regulated through epigenetics. Disturbances related to endometrial GLUTs have been linked with gestational diabetes, infertility, and polycystic ovarian syndrome.[26] GLUT1 is present in endothelial cells of the blood–brain barrier and is upregulated in case of a hypoglycemic crisis. The expression by translocation of GLUT1 increases five-fold in contrast to 20 20-fold increase in GLUT4 in response to upregulation by insulin.[27]
GLUT2 functions as the only GLUT that can mediate the bidirectional flow of glucose from the lumen to portal circulation and vice versa depending on the blood glucose levels, this characteristic is mainly due to its ability to be translocated to intestinal brush border cell in response to dietary sugar intake.[28] Glucose and fructose enter the portal circulation by GLUT2 transporters present on the basolateral membrane of intestinal epithelium.[29] In response to the fasting state, GLUT2 also carries out the release of glucose after hepatic gluconeogenesis. It also plays a role in glucose reabsorption along the basolateral border of renal epithelium.[30]
A duplicon of GLUT3 named GLUT14 has also been purified which is predominantly expressed in human testis and also shares a unique association with inflammatory bowel disease, especially Crohn’s disease.[31,32] GLUT3 present in endometrium also plays a crucial role alongside GLUT1 in decidualization and differentiation of endometrial stroma during the secretory phase of menstrual cycle, hence providing a nutritive environment for the implantation of zygote.[33]
GLUT4 protein has always been a subject of interest for researchers mainly due to its property of being the cardinal insulin-sensitive transporter in the SLC2A family. In response to insulin exposure, they are translocated from an intracellular pool to the plasma membrane in adipocytes and sarcolemmal membrane and T-tubules of muscle cells.[34] GLUT4 is also responsible for glucose transport in muscles during strenuous exercise.[35]
GLUT5 is recommended as an additional hexose transporter system present in the brush border of intestinal epithelium alongside SGLT.[36] It shows an increase in expression in response to dietary fructose stimulation.[37] Gene expression for GLUT5 is also found to be upregulated in non-insulin-dependent diabetes mellitus (NIDDM). This finding has further warranted the use of GLUT5 inhibitors in antidiabetic therapy.[38]
GLUT6 human isoform was replicated from leukocytes on account of its ability to over-express in inflammatory and endothelial cells in response to viral load.[39] In the year 2000, it was then referred to as GLUT9, and the encoding gene for this protein was later termed SLC2A6; hence, the name was changed to GLUT6 by the HUGO Gene Nomenclature Committee.[40]
GLUT7 was cloned from intestinal complementary deoxyribonucleic acid in the year 2004 using a PCR-based strategy. It was the last SLC2A family member to be cloned using this technique.[41] GLUT8 plays a role in blastocyst where it is upregulated in response to insulin, thereby, functionally acting as GLUT4, which is absent in the early stages of embryonic development.[42] GLUT9 is a class 2 GLUT and plays a crucial role in urate reabsorption.[43] GLUT10 has its encoding gene on chromosome 20q12-q31, a locus also associated with non-insulin-dependent diabetes mellitus. Therefore, GLUT10 has become an interesting center of attention for the development of more insight and therapeutic approaches for type-2 diabetes mellitus. Mutation in the gene encoding for GLUT10, present in vascular smooth muscle cells, is associated with stenosis and tortuosity of large vessels causing arterial tortuosity syndrome.[44]
In humans, three isoforms of GLUT11 are found due to the presence of splice variants. GLUT11-A, GLUT11-B, and GLUT11-C have been cloned, each with a distinct N-terminal sequence. They share the same transporter activity for fructose and glucose excluding galactose.[45] GLUT12 is released in response to insulin where its upregulation occurs from intracellular cytoplasmic reservoir to the lipid bilayer in insulin-sensitive adipose tissue, skeletal muscle, and heart.[46]
GLUT13, also known as HMIT, is responsible for proton-dependent electrogenic transport associated with the translocation of GLUT13 to the cell membrane following cell depolarization, raised calcium influx, and protein kinase C activation [Table 2 and Figure 3].
| GLUTs | Amino acids | Molecular weight | Substrate, Km | Class | Reference |
|---|---|---|---|---|---|
| GLUT1 | 492 | 54 kDa | Glucose, ~6.9 mM | 1 | [83] |
| GLUT2 | 524 | 60–62 kDa | Glucose, ~17 mM Galactose, ~92 mM Mannose, ~125 mM Fructose, ~76 mM Glucosamine, ~0.8 mM |
1 | [10,84] |
| GLUT3 | 496 | 54 kDa | Glucose, ~1.6 mM | 1 | [85] |
| GLUT4 | 509 | 55 kDa | Glucose, ~5–6 mM | 1 | [86] |
| GLUT5 | 501 | 55 kDa | Fructose, ~6 mM | 2 | [87] |
| GLUT6 | 507 | 55 kDa | Glucose | 3 | [39] |
| GLUT7 | 524 | 55 kDa | Fructose, ~0.06 mM Glucose, ~0.3 mM |
2 | [41] |
| GLUT8 | 477 | 51.5 kDa | Glucose Trehalose (in hepatocytes) |
3 | [88-90] |
| GLUT9 | SLC2A9a- 540 SLC2A9b- 512 |
59 kDa 55 kDa |
Glucose, ~0.61 mM Fructose, ~0.42 mM Urate |
2 | [42,43,91] |
| GLUT10 | 541 | 56 kDa | Glucose, ~0.3 mM | 3 | |
| GLUT11 | 503 496 |
42 kDa | Fructose Glucose |
2 | [23,92] |
| GLUT12 | 617 | 50 kDa | Glucose | 3 | [46] |
| GLUT13 | 629 | 75–90 kDa | Myo-inositol | 3 | [22] |
GLUTs: Glucose transporters
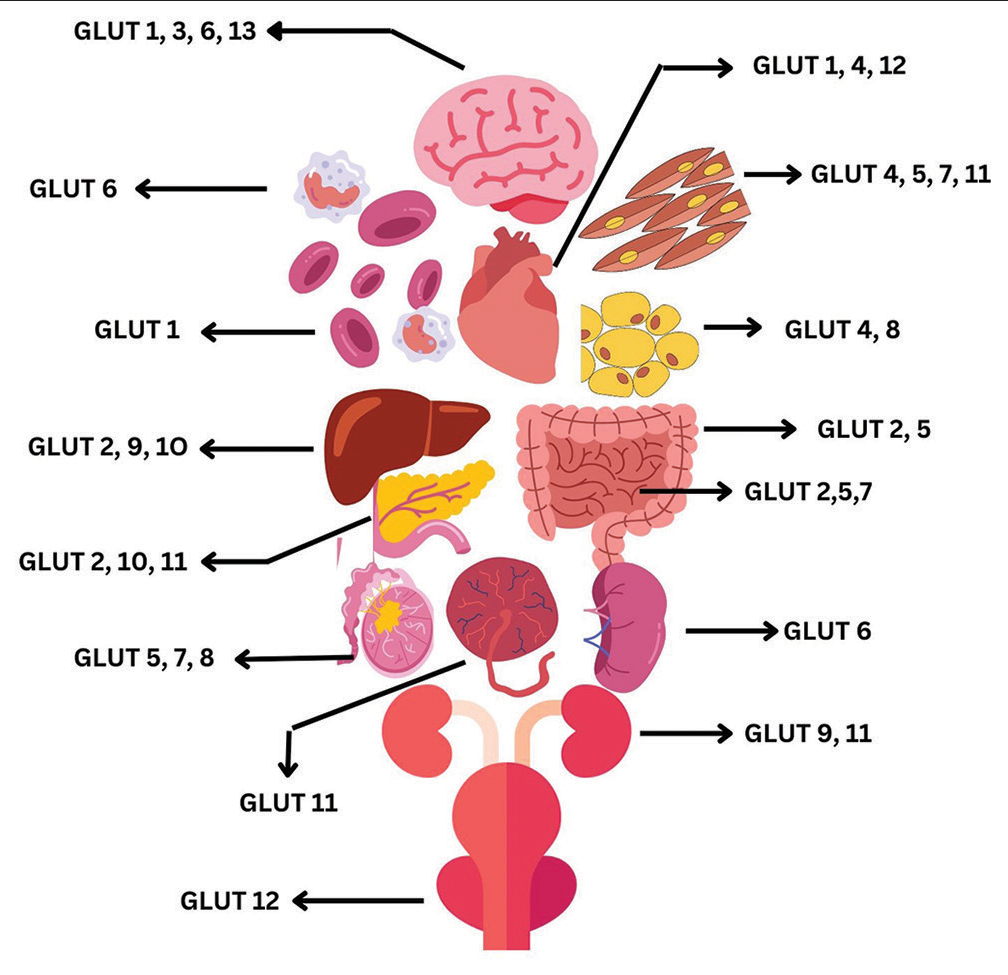
- Expression of GLUTs in the different organs and tissues of the body. The image was designed using Canva. GLUTs: Glucose transporters.
PATHOLOGICAL IMPLICATIONS OF GLUTs
This family of transporters plays a crucial role in hexose metabolism and studies have shown a number of diseased states where the alteration in the pattern of expression of this family of transporters has evidently helped in the diagnosis and treatment of different ailments. This section sheds light on the link of GLUT proteins with different clinical disorders along with some specific GLUT-associated genetic diseases.
Cancer
GLUT1 along with GLUT3 is over-expressed in many neoplasia including brain,[47,48] breast, and cervical cancers.[49] GLUT1 and GLUT4[50] show an increased expression in colon,[51] kidney, ovary,[52] prostate,[53] skin,[54] and thyroid cancers.[55] Cancers with an overexpression of GLUT1 are deemed more aggressive. Due to the ubiquitous distribution of this protein, many therapeutic approaches have been made to target this transporter, but the same widespread prevalence also hinders achieving treatment specificity.[56] Recurrence and aggressiveness of brain tumors, especially glioblastoma, are also associated with an over-expressed GLUT3.[48] GLUT5 overexpression is linked with a number of cancers, especially ovarian carcinoma[57] and their metastatic potential. This feature makes it an easy aim for various chemotherapeutic approaches.[58] GLUT12 (along with GLUT1) is sensitive to stimulation by androgens in the prostate gland; hence, an overexpression of these proteins is observed in prostate cancers.[59] GLUT12 was cloned from Michigan Cancer Foundation (MCF)7, a human breast cancer cell line, showing strong positive immunohistochemical staining in ductal cell carcinoma in situ than in benign ducts of breast cancer tissue.[60] Eight out of ten invasive cancers showed an over-expression of GLUT12.[61]
Metabolic syndrome and diabetes
The liver expresses a host of GLUTs including GLUT1, 2, 5, 8, and 9 with GLUT2 being the main transporter of glucose inside hepatocytes. Studies have found a strong correlation between obesity, type 2 diabetes, and non-alcoholic fatty liver disease with impaired glucose homeostasis.[62] GLUT4 shares a tight association with metabolic syndromes such as obesity and type 2 diabetes, strongly depicting an altered expression in adipose tissue, especially in patients presenting with type 2 diabetes and insulin resistance.[63] Selective insulin resistance in aging HepG2 cells coupled with changes in GLUT4 expression points toward an association of GLUT4 with liver cirrhosis.[64] GLUT5 is also responsible for fructose-mediated metabolic syndrome since its metabolites deplete ATP and cause oxidative stress amongst soft tissues leading to an inflammatory cascade in the liver, adipocytes, pancreas, and kidneys.[65] The association of increased fructose consumption with early-onset metabolic syndrome has directed the attention of researchers toward the inhibition of class 2 fructose transporters as a mode of therapy for the mentioned disorder.[66]
GLUT1 deficiency syndrome (G1DS)
G1DS is an autosomal dominant mutation in the SLC2A1 gene. This haploinsufficiency mutation can cause hypoglycorrhachia (decreased concentration of glucose in cerebrospinal fluid), convulsions, developmental delay, and cerebral atrophy.[67] However, the disease complications can be treated with a ketogenic diet that bypasses the need for glucose uptake by GLUT1.[68]
Fanconi–Bickel syndrome
Fanconi–Bickel syndrome is a rare disorder of GLUT2 and presents as autosomal recessive glycogen storage disease with three mutations found in the SLC2A2 gene, causing loss of function in the abnormally shortened transport proteins. The disease is presented with transient post-prandial hyperglycemia, followed by hypoglycemia, mainly due to hepatorenal glycogen accumulation and impaired transport of glucose and galactose. Defective absorption of hexose in enterocytes is also responsible for the occurrence of diarrhea in this disorder. Impaired reabsorption of glucose in renal tubular cells and its loss in the urine (glucosuria) is caused by Fanconi Nephropathy, a condition that further aggravates hypoglycemia.[69]
GLUT9 disorder associated with urate metabolism
Organic anion urate appears to be the primary substrate for GLUT9 protein. It maintains serum urate level within a narrow range of 250–300 μM by its action on hepatocytes and the basolateral membrane of the renal proximal convoluted tubule. A defect in GLUT9 can cause hypouricemia which can be further worsened by uric acid stones.[70] GLUT9 along with urate transporter 1 works collectively in the maintenance of uric acid homeostasis associated with articular cartilage and chondrocytes, any defective polymorphism in this transporter can also cause hyperuricemia, further leading to chronic gouty arthritis.[71]
Arterial tortuosity syndrome
Arterial tortuosity syndrome is an autosomal recessive disorder associated with a mutation in one of the genes encoding for human GLUT10. The vessels deficient in GLUT10 show an increased activity of transforming growth factor-β (TGFβ) causing a disarray of elastic fibers in tunica media, hence causing tortuous and stenotic arteries with an increased propensity of aneurysm formation.[44] Increased expression of TGFβ in GLUT10 deficiency can also be linked with microangiopathic changes seen in type 2 diabetes and provides evidence to further work on TGFβ-targeted treatment strategies.
THERAPEUTIC ROLES OF GLUTS
The role played by GLUT1 in glucose utilization by autoimmune T-cells is being used pharmacologically to inhibit their cellular metabolism and render them unable to damage beta-islet cells as a treatment modality for type 1 diabetes mellitus.[72] Development of chemotherapeutics that selectively target GLUT1 in plasmodium-infested red blood cells. They starve off the parasite by cutting off the glucose supply. This is currently underway as a treatment for Malaria.[73] GLUT2 present in intestinal absorption of hexoses has been a target of interest employed in many antidiabetic treatments.[74] Treatment with GLUT5 inhibitor pioglitazone has been shown to reverse the upregulation of GLUT5 seen in skeletal muscle cells of NIDDM. An antidiabetic drug tiliroside has also been shown to inhibit glucose uptake in gastrointestinal tract (GIT).[75] A non-specific GLUT inhibitor ritonavir has proven to be beneficial against myocardial necrosis caused by catecholamines.[76] N-[4-(methylsulfonyl)-2-nitrophenyl]-1,3-benzodioxol-5-amine (MSNBA) is a highly selective competitive GLUT5 inhibitor used in breast cancer due to its propensity of impeding fructose transport meanwhile causing no effect on the transport of fructose by GLUT2 or glucose transport by GLUT1–4.[77]
Warburg effect
The cancerous cells in the human body have adapted to use glycolysis as their main mode of metabolism; this causes an increased expression of GLUT proteins on the cell membrane of neoplastic cells. This metabolic adaptation known as the Warburg Effect creates a negative energy balance.[78] The Warburg effect plays an important role in directing the anti-cancer treatment toward increased glycolysis and upregulation of GLUTs. Various regimens aiming to downregulate selected GLUTs have proven as a beneficial chemotherapeutic strategy, WZB117 is a prototype drug currently under development and can be used as a potential chemotherapeutic agent in GLUT1-specific cancers.[79]
Another strategic treatment for cancers, exploiting GLUT-mediated glucose transport and metabolism, is cell starvation. Cell starvation decreases the viability of cancer cells.[80] Starvation-dependent differential stress resistance aims to protect normal cells against chemotherapy and helps them preserve their normal morphology and function.[81] GLUT5-specific inhibitors in cancers with increased fructose demand and the production of fructose-specific probes for imaging studies have paved a new path in the development of different strategies for cancer cure and prevention.[82] However, detailed modeling strategies are required to design analogs only targeting GLUT5 and exempting GLUT2 transporters (also have an affinity for fructose).[77]
CONCLUSION
To this date, 13 isoforms of GLUTs have been isolated with substantial research having been conducted on the structure-, function-, and tissue-specific distribution of each GLUT family member. Their role in transporting glucose across the membrane is indispensable for executing vital chemical reactions required for sustainability of normal body functions and any mutation in their normal genomics can lead to deficiency syndromes associated with the respective transporter. However, more evidence-based research studies and clinical trials are required to elucidate their significance in the treatment and diagnosis of ailments such as metabolic syndrome, diabetes, and cancer which can lead to a remarkable revolution in clinical medicine.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The author(s) confirms that there was no use of Artificial Intelligence (AI)-Assisted Technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Sugar for the Brain: The Role of Glucose in Physiological and Pathological Brain Function. Trends Neurosci. 2013;36:587-97.
- [CrossRef] [PubMed] [Google Scholar]
- A Call for Systematic Research on Solute Carriers. Cell. 2015;162:478-87.
- [CrossRef] [PubMed] [Google Scholar]
- Glucose Transport Families SLC5 and SLC50. Mol Aspects Med. 2013;34:183-96.
- [CrossRef] [PubMed] [Google Scholar]
- Sodium-Glucose Cotransporters: Functional Properties and Pharmaceutical Potential. J Diabetes Investig. 2020;11:770-82.
- [CrossRef] [PubMed] [Google Scholar]
- The Sodium/Substrate Symporter Family: Structural and Functional Features. FEBS Lett. 2002;529:73-7.
- [CrossRef] [PubMed] [Google Scholar]
- Plant SWEET Family of Sugar Transporters: Structure, Evolution, and Biological Functions. Biomolecules. 2022;12:205.
- [CrossRef] [PubMed] [Google Scholar]
- Glucose Transporter 1 in Health and Disease. J Oral Maxillofac Pathol. 2019;23:443-9.
- [CrossRef] [PubMed] [Google Scholar]
- Major Facilitator Superfamily. Microbiol Mol Biol Rev. 1998;62:1-34.
- [CrossRef] [PubMed] [Google Scholar]
- Functional Properties and Genomics of Glucose Transporters. Curr Genomics. 2007;8:113-28.
- [CrossRef] [PubMed] [Google Scholar]
- Will the Original Glucose Transporter Isoform Please Stand Up! Am J Physiol Endocrinol Metab. 2009;297:E836-48.
- [CrossRef] [PubMed] [Google Scholar]
- Crystal Structure of a Bacterial Homologue of Glucose Transporters GLUT1-4. Nature. 2012;490:361-6.
- [CrossRef] [PubMed] [Google Scholar]
- Glucose Transporters (GLUT and SGLT): Expanded Families of Sugar Transport Proteins. Br J Nutr. 2003;89:3-9.
- [CrossRef] [PubMed] [Google Scholar]
- Characterizing Hydropathy of Amino Acid Side Chain in a Protein Environment by Investigating the Structural Changes of Water Molecules Network. Front Mol Biosci. 2021;8:626837.
- [CrossRef] [PubMed] [Google Scholar]
- Molecular Biology of Mammalian Glucose Transporters. Diabetes Care. 1990;13:198-208.
- [CrossRef] [PubMed] [Google Scholar]
- Regulation of Glucose-Transporter Function. Diabetes Care. 1990;13:219-27.
- [CrossRef] [PubMed] [Google Scholar]
- The Extended GLUT-Family of Sugar/ Polyol Transport Facilitators: Nomenclature, Sequence Characteristics, and Potential Function of its Novel Members (review) Mol Membr Biol. 2001;18:247-56.
- [CrossRef] [PubMed] [Google Scholar]
- Structure of, and Functional Insight into the GLUT Family of Membrane Transporters. Cell Health Cytoskeleton. 2015;7:167.
- [CrossRef] [Google Scholar]
- A Highly Conserved Hydrophobic Motif in the Exofacial Vestibule of Fructose Transporting SLC2A Proteins Acts as a Critical Determinant of their Substrate Selectivity. Mol Membr Biol. 2007;24:455-63.
- [CrossRef] [PubMed] [Google Scholar]
- SLC2A9 is a High-Capacity Urate Transporter in Humans. PLoS Med. 2008;5:e197.
- [CrossRef] [PubMed] [Google Scholar]
- Identification of a Mammalian H(+)-MyoInositol Symporter Expressed Predominantly in the Brain. EMBO J. 2001;20:4467-77.
- [CrossRef] [PubMed] [Google Scholar]
- Characterization of the Human SLC2A11 (GLUT11) Gene: Alternative Promoter usage, Function, Expression, and Subcellular Distribution of Three Isoforms, and Lack of Mouse Orthologue. Mol Membr Biol. 2005;22:339-51.
- [CrossRef] [PubMed] [Google Scholar]
- Reconstitution and Purification of the D-Glucose Transporter from Human Erythrocytes. J Biol Chem. 1977;252:7384-90.
- [CrossRef] [PubMed] [Google Scholar]
- Quantitative Analysis of Glucose Transporter mRNAs in Endometrial Stromal Cells Reveals Critical Role of GLUT1 in Uterine Receptivity. Endocrinology. 2011;152:2123-8.
- [CrossRef] [PubMed] [Google Scholar]
- Endometrial Glucose Transporters in Health and Disease. Front Cell Dev Biol. 2021;9:703671.
- [CrossRef] [PubMed] [Google Scholar]
- Cell Surface Labeling of Glucose Transporter Isoform GLUT4 by Bis-Mannose Photolabel. Correlation with Stimulation of Glucose Transport in rat Adipose Cells by Insulin and Phorbol Ester. J Biol Chem. 1990;265:18172-9.
- [CrossRef] [PubMed] [Google Scholar]
- The Facilitated Component of Intestinal Glucose Absorption. J Physiol. 2001;531:585-95.
- [CrossRef] [PubMed] [Google Scholar]
- GLUT2 is the Transporter for Fructose Across the Rat Intestinal Basolateral Membrane. Gastroenterology. 1993;105:1050-6.
- [CrossRef] [PubMed] [Google Scholar]
- Direct and Indirect Actions of Insulin: Role of Insulin Receptor, Glucose Transporters (GLUTs), and Sodium-Glucose Linked Transporters (SGLTs) In: Gupta A, ed. Understanding Insulin and Insulin Resistance. Netherlands: Elsevier; 2022. p. :179-201. Ch. 6
- [CrossRef] [Google Scholar]
- Transcript Analysis of Zebrafish GLUT3 Genes, slc2a3a and slc2a3b, Define Overlapping as Well as Distinct Expression Domains in the Zebrafish (Danio rerio) Central Nervous System. Front Mol Neurosci. 2019;12:199.
- [CrossRef] [PubMed] [Google Scholar]
- GLUT14, a Duplicon of GLUT3, is Specifically Expressed in Testis as Alternative Splice Forms. Genomics. 2002;80:553-7.
- [CrossRef] [PubMed] [Google Scholar]
- Glucose Transporter Proteins (GLUT) in Human Endometrium: Expression, Regulation, and Function throughout the Menstrual Cycle and in Early Pregnancy. J Clin Endocrinol Metab. 2003;88:3885-92.
- [CrossRef] [PubMed] [Google Scholar]
- Kinetic Evidence for Unique Regulation of GLUT4 Trafficking by Insulin and AMP-Activated Protein Kinase Activators in L6 Myotubes. J Biol Chem. 2010;285:1653-60.
- [CrossRef] [PubMed] [Google Scholar]
- Human Facilitative Glucose Transporters, Isolation, Functional Characterization, and Gene Localization of cDNAs Encoding an Isoform (GLUT5) Expressed in Small Intestine, Kidney, Muscle, and Adipose Tissue and an Unusual Glucose Transporter Pseudogene-Like Sequence (GLUT6) J Biol Chem. 1990;265:13276-82.
- [CrossRef] [PubMed] [Google Scholar]
- Regulation of the Fructose Transporter GLUT5 in Health and Disease. Am J Physiol Endocrinol Metab. 2008;295:E227-37.
- [CrossRef] [PubMed] [Google Scholar]
- Overexpression of GLUT5 in Diabetic Muscle is Reversed by Pioglitazone. Diabetes Care. 2007;30:925-31.
- [CrossRef] [PubMed] [Google Scholar]
- Upregulation of Glucose Uptake and Hexokinase Activity of Primary Human CD4+ T Cells in Response to Infection with HIV-1. Viruses. 2018;10:114.
- [CrossRef] [PubMed] [Google Scholar]
- Activity and Genomic Organization of Human Glucose Transporter 9 (GLUT9), a Novel Member of the Family of Sugar-Transport Facilitators Predominantly Expressed in Brain and Leucocytes. Biochem J. 2000;350(Pt 3):771-6.
- [CrossRef] [PubMed] [Google Scholar]
- Cloning and Functional Characterization of the Human GLUT7 Isoform SLC2A7 from the Small Intestine. Am J Physiol Gastrointest Liver Physiol. 2004;287:G236-42.
- [CrossRef] [PubMed] [Google Scholar]
- GLUT8 is a Glucose Transporter Responsible for Insulin-Stimulated Glucose Uptake in the Blastocyst. Proc Natl Acad Sci U S A. 2000;97:7313-8.
- [CrossRef] [PubMed] [Google Scholar]
- Expression of SLC2A9 Isoforms in the Kidney and their Localization in Polarized Epithelial Cells. PLoS One. 2014;9:e84996.
- [CrossRef] [PubMed] [Google Scholar]
- Mutations in the Facilitative Glucose Transporter GLUT10 Alter Angiogenesis and Cause Arterial Tortuosity Syndrome. Nat Genet. 2006;38:452-7.
- [CrossRef] [PubMed] [Google Scholar]
- Molecular Cloning of a Member of the Facilitative Glucose Transporter Gene Family GLUT11 (SLC2A11) and Identification of Transcription Variants. Biochem Biophys Res Commun. 2001;289:1218-24.
- [CrossRef] [PubMed] [Google Scholar]
- Insulin-Stimulated Translocation of Glucose Transporter (GLUT) 12 Parallels that of GLUT4 in Normal Muscle. J Clin Endocrinol Metab. 2009;94:3535-42.
- [CrossRef] [PubMed] [Google Scholar]
- Expression of GLUT1 in Pseudopalisaded and Perivascular Tumor Cells is an Independent Prognostic Factor for Patients with Glioblastomas. J Neuropathol Exp Neurol. 2019;78:389-97.
- [CrossRef] [PubMed] [Google Scholar]
- A Role for GLUT3 in Glioblastoma Cell Invasion that is not Recapitulated by GLUT1. Cell Adh Migr. 2021;15:101-15.
- [CrossRef] [PubMed] [Google Scholar]
- Expression of GLUT1 and GLUT3 Glucose Transporters in Endometrial and Breast Cancers. Pathol Oncol Res. 2012;18:721-8.
- [CrossRef] [PubMed] [Google Scholar]
- Immunohistochemical Localization of Glucose Transporters in Human Renal Cell Carcinoma. J Urol. 1995;153:798-801.
- [CrossRef] [PubMed] [Google Scholar]
- GLUT1 Glucose Transporter Expression in Colorectal Carcinoma: A Marker for Poor Prognosis. Cancer. 1998;83:34-40.
- [CrossRef] [Google Scholar]
- Ovarian Cancer Relies on Glucose Transporter 1 to Fuel Glycolysis and Growth: Anti-Tumor Activity of BAY-876. Cancers (Basel). 2018;11:33.
- [CrossRef] [PubMed] [Google Scholar]
- GLUT1 Expression in High-Risk Prostate Cancer: Correlation with (18)F-FDG-PET/CT and Clinical Outcome. Prostate Cancer Prostatic Dis. 2020;23:441-8.
- [CrossRef] [PubMed] [Google Scholar]
- GLUT-1 Expression in Cutaneous Basal and Squamous Cell Carcinomas. Int J Surg Pathol. 2015;23:447-53.
- [CrossRef] [PubMed] [Google Scholar]
- GLUT1 Glucose Transporter Expression in Benign and Malignant Thyroid Nodules. Thyroid. 1997;7:363-7.
- [CrossRef] [PubMed] [Google Scholar]
- Glucose Transporters as a Target for Anticancer Therapy. Cancers (Basel). 2021;13:4184.
- [CrossRef] [PubMed] [Google Scholar]
- GLUT5 Increases Fructose Utilization in Ovarian Cancer. Onco Targets Ther. 2019;12:5425-36.
- [CrossRef] [PubMed] [Google Scholar]
- GLUT5 (SLC2A5) Enables Fructose-Mediated Proliferation Independent of Ketohexokinase. Cancer Metab. 2021;9:12.
- [CrossRef] [PubMed] [Google Scholar]
- Expression and Localization of GLUT1 and GLUT12 in Prostate Carcinoma. Cancer. 2003;97:2035-42.
- [CrossRef] [PubMed] [Google Scholar]
- Potential Roles of GLUT12 for Glucose Sensing and Cellular Migration in MCF-7 Human Breast Cancer Cells Under High Glucose Conditions. Anticancer Res. 2017;37:6715-22.
- [CrossRef] [Google Scholar]
- Differential Expression of GLUT12 in Breast Cancer and Normal Breast Tissue. Cancer Lett. 2003;193:225-33.
- [CrossRef] [PubMed] [Google Scholar]
- Glucose Transporters in Adipose Tissue, Liver, and Skeletal Muscle in Metabolic Health and Disease. Pflugers Arch. 2020;472:1273-98.
- [CrossRef] [PubMed] [Google Scholar]
- Human Subcutaneous Adipose Tissue Glut 4 mRNA Expression in Obesity and Type 2 Diabetes. Acta Diabetol. 2013;50:227-32.
- [CrossRef] [PubMed] [Google Scholar]
- Selective Insulin Resistance in Hepatocyte Senescence. Exp Cell Res. 2015;331:38-45.
- [CrossRef] [PubMed] [Google Scholar]
- High Dietary Fructose: Direct or Indirect Dangerous Factors Disturbing Tissue and Organ Functions. Nutrients. 2017;9:335.
- [CrossRef] [PubMed] [Google Scholar]
- Dietary Fructose, Salt Absorption and Hypertension in Metabolic Syndrome: Towards a New Paradigm. Acta Physiol (Oxf). 2011;201:55-62.
- [CrossRef] [PubMed] [Google Scholar]
- Glut1 Deficiency Syndrome and Erythrocyte Glucose Uptake Assay. Ann Neurol. 2011;70:996-1005.
- [CrossRef] [PubMed] [Google Scholar]
- Defective Glucose Transport across the Blood-Brain Barrier as a cause of Persistent Hypoglycorrhachia, Seizures, and Developmental Delay. N Engl J Med. 1991;325:703-9.
- [CrossRef] [PubMed] [Google Scholar]
- Mutations in GLUT2, the Gene for the Liver-Type Glucose Transporter, in Patients with FanconiBickel Syndrome. Nat Genet. 1997;17:324-6.
- [CrossRef] [PubMed] [Google Scholar]
- Homozygous SLC2A9 Mutations cause Severe Renal Hypouricemia. J Am Soc Nephrol. 2010;21:64-72.
- [CrossRef] [PubMed] [Google Scholar]
- Urate Transport Capacity of Glucose Transporter 9 and Urate Transporter 1 in Cartilage Chondrocytes. Mol Med Rep. 2019;20:1645-54.
- [CrossRef] [PubMed] [Google Scholar]
- Pharmacological Targeting of GLUT1 to Control Autoreactive T Cell Responses. Int J Mol Sci. 2019;20:4962.
- [CrossRef] [PubMed] [Google Scholar]
- Structural Basis for Blocking Sugar Uptake into the Malaria Parasite Plasmodium falciparum. Cell. 2020;183:258-68.e12.
- [CrossRef] [PubMed] [Google Scholar]
- Identification of New GLUT2-Selective Inhibitors through in silico Ligand Screening and Validation in Eukaryotic Expression Systems. Sci Rep. 2021;11:13751.
- [CrossRef] [PubMed] [Google Scholar]
- Tiliroside, a Glycosidic Flavonoid, Inhibits Carbohydrate Digestion and Glucose Absorption in the Gastrointestinal Tract. Mol Nutr Food Res. 2012;56:435-45.
- [CrossRef] [PubMed] [Google Scholar]
- Cardioprotective Effect of Ritonavir, an Antiviral Drug, in Isoproterenol Induced Myocardial Necrosis: A New Therapeutic Implication. J Transl Med. 2013;11:80.
- [CrossRef] [PubMed] [Google Scholar]
- Discovery of a Specific Inhibitor of Human GLUT5 by Virtual Screening and in vitro Transport Evaluation. Sci Rep. 2016;6:24240.
- [CrossRef] [PubMed] [Google Scholar]
- Warburg Effect Revisited: Merger of Biochemistry and Molecular Biology. Science. 1981;213:303-7.
- [CrossRef] [PubMed] [Google Scholar]
- A Small-Molecule Inhibitor of Glucose Transporter 1 Downregulates Glycolysis, Induces Cell-Cycle Arrest, and Inhibits Cancer Cell Growth in vitro and in vivo. Mol Cancer Ther. 2012;11:1672-82.
- [CrossRef] [PubMed] [Google Scholar]
- Glucose Deprivation Activates a Metabolic and Signaling Amplification Loop Leading to Cell Death. Mol Syst Biol. 2012;8:589.
- [CrossRef] [PubMed] [Google Scholar]
- Starvation-Dependent Differential Stress Resistance Protects Normal but not Cancer Cells against High-Dose Chemotherapy. Proc Natl Acad Sci U S A. 2008;105:8215-20.
- [CrossRef] [PubMed] [Google Scholar]
- Synthesis and Evaluation of Fructose Analogues as Inhibitors of the D-Fructose Transporter GLUT5. Bioorg Med Chem. 2000;8:1825-33.
- [CrossRef] [PubMed] [Google Scholar]
- Blood-Brain Barrier Glucose Transporter: Effects of Hypo-and Hyperglycemia Revisited. J Neurochem. 1999;72:238-47.
- [CrossRef] [PubMed] [Google Scholar]
- GLUT2 is a High Affinity Glucosamine Transporter. FEBS Lett. 2002;524:199-203.
- [CrossRef] [PubMed] [Google Scholar]
- Distribution of GLUT3 Glucose Transporter Protein in Human Tissues. Biochem Biophys Res Commun. 1992;188:149-54.
- [CrossRef] [PubMed] [Google Scholar]
- Current understanding of glucose transporter 4 expression and functional mechanisms. World J Biol Chem. 2020;11:76-98.
- [CrossRef] [PubMed] [Google Scholar]
- Fructose Transporter in Human Spermatozoa and Small Intestine is GLUT5. J Biol Chem. 1992;267:14523-6.
- [CrossRef] [PubMed] [Google Scholar]
- GLUT1 and GLUT8 Support Lactose Synthesis in Golgi of Murine Mammary Epithelial Cells. J Physiol Biochem. 2019;75:209-15.
- [CrossRef] [PubMed] [Google Scholar]
- SLC2A8 (GLUT8) is a Mammalian Trehalose Transporter Required for Trehalose-Induced Autophagy. Sci Rep. 2016;6:38586.
- [CrossRef] [PubMed] [Google Scholar]
- GLUT8, a Novel Member of the Sugar Transport Facilitator Family with Glucose Transport Activity. J Biol Chem. 2000;275:16275-80.
- [CrossRef] [PubMed] [Google Scholar]
- Cloning and Expression Analysis of a Novel Member of the Facilitative Glucose Transporter Family, SLC2A9 (GLUT9) Genomics. 2000;66:217-20.
- [CrossRef] [PubMed] [Google Scholar]
- Characterization of Human Glucose Transporter (GLUT) 11 (encoded by SLC2A11), a Novel Sugar-Transport Facilitator Specifically Expressed in Heart and Skeletal Muscle. Biochem J. 2001;359:443-9.
- [CrossRef] [PubMed] [Google Scholar]







