Translate this page into:
Incidence of Ventilator-Associated Pneumonia and its Bacterial Characterization – Intervention Based Prospective Study

*Corresponding author: Dr. Kalaivani Ramakrishnan, Professor, Departmentof Microbiology, Mahatma Gandhi Medical College and Research Institute, Sri Balaji Vidhyapeeth - Deemed to be University, Puducherry, India. kalaimicro21@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Ramakrishnan K, Jahagirdar SN, Ravisankar M, Seetha K. Incidence of Ventilator-Associated Pneumonia and its Bacterial Characterization – Intervention Based Prospective Study. Glob J Med Pharm Biomed Update 2023;18:27.
Abstract
Objectives:
Ventilator-associated pneumonia (VAP) is a widely recognized and potentially fatal healthcare-related infection that occurs in all high-dependency units. Mechanically ventilated patients are at an elevated risk of developing VAP, which has a high death and morbidity rate. The prevalence of VAP varies greatly depending on the location and diagnostic approach. Radiological and clinical markers impact VAP diagnosis accuracy. Reliable sampling and confirmation of microbes are highly recommended. The purpose of this study was to document the incidence, patient distribution, bacteriological profile, and antibiotic susceptibility pattern of VAP patients.
Material and Methods:
A prospective observational study was done between January 2016 and December 2019. Critically, ill patients on mechanical ventilation for more than 48 hours were included in the study. Based on the initial baseline, positive end-expiratory pressure, and fraction of inspired oxygen were followed by three-tier VAP criteria as per NSHN guidelines.
Results:
Out of 1220 VAP-suspected patients (mechanically ventilated), 49 patients developed hospital-acquired VAP. The incidence of VAP significantly reduced from 10.7 to 1.4 VAP/1000 ventilator days with continuous intervention and auditing over some time. Elderly males aged 51–66 years were found to be in higher risk groups. Klebsiella pneumoniae and Pseudomonas aeruginosa were found to be the most common pathogen. The majority of Enterobacterales (79%) were found to be resistant to third-generation cephalosporin, 69% were resistant toward fluoroquinolone and cotrimoxazole, followed by 55% resistance to beta-lactam and beta-lactamase inhibitor combination.
Conclusion:
Targeted strategies with implementable policies, such as the care bundle approach, will reduce the in-patient days. It might improve patient outcomes and reduce the incidence of VAP.
Keywords
Ventilator-associated pneumonia
Bacterial pneumonia
Mechanical ventilation
pulmonary infections
Bacterial isolates
Ventilator-associated event
INTRODUCTION
Infections contracted in hospitals or healthcare-associated infections (HAI) raise morbidity and mortality dramatically, particularly in underdeveloped countries.[1] They cause a significant emotional impact among family members as well as patients and increase the length of hospital stay, and escalate the cost of healthcare expenditure[2,3] Hand hygiene, a lack of trained personnel for infection control and prevention, a lack of accessibility or knowledge on the proper use of personal protective equipment (PPE), inadequate biomedical waste management policies and perception, widespread antimicrobial agent misuse, and other factors contribute to an increase in the HAI rate.[4]
Ventilator-associated pneumonia (VAP) is one of the most prevalent HAIs among critically ill patients on mechanical ventilation.[3] VAP has been defined as lung parenchyma infection in a patient who has been on invasive mechanical ventilation for more than 2 calendar days.[5,6] The prevalence of VAP ranges from 6% to 52%, with some cases reaching 76%. The probability of developing VAP in intensive care patients is estimated to be 3%/day for the first 5 days of mechanical breathing, 2%/day for the next 5–10 days, and 1%/day after that.[7] Even with further clinical criteria revisions, the diagnostic criteria for VAP remain contentious. The current surveillance study aimed to document the incidence, patient distribution, bacteriological profile, and antibiotic susceptibility pattern of patients who developed VAP during the study period using guidelines from the Centers for Disease Control and Prevention-National Healthcare Safety Network (CDC NHSN).[8]
MATERIAL AND METHODS
This prospective study was conducted between January 2016 and December 2019 from a tertiary care hospital, in Puducherry. Since this is an audit-based study for quality improvement, sample size estimation was not done. A universal sampling method was adopted to include all eligible study candidates over a period of 4 years as per protocol. All adult patients (>18 years) admitted in the critical care unit (CCU) requiring mechanical ventilator support for more than 2 calendar days were recruited in this study. The Institute Human Ethical Committee clearance was obtained (IHEC – MGMCRI Faculty – 2014–40). Those patients who were intubated/mechanically ventilated outside this hospital and patients with evidence of respiratory tract infection before intubation/within 2 days of admission, patients who expired, if they developed pneumonia within 48 hours or those who were admitted with pneumonia at the time of admission and patients population with acute respiratory distress syndrome were excluded from the study. Patients’ demographic details, date of CCU admission, indication for ventilation, date of respiratory samples sent for culture, and susceptibility testing reports were recorded.
The study duration was divided into two phases, that is, phase 1: (2016–2017) before intervention and phase 2: (2018–2019) after intervention. During both phases, ventilator settings, mode, alterations in the settings, patients’ daily vitals, oxygen saturation, the addition of new antibiotics, and respiratory secretions culture reports were monitored regularly. Based on the CDC ventilator-associated event (VAE) surveillance criteria, the diagnosis of VAP was made. The three-tier indicators are as follows: Ventilator-associated complications (VACs), infection-related ventilator-associated complications (IVAC), and possible/probable VAP. The first step of VAE is VAC which will identify any complication occurring in mechanically ventilated patients regardless of the origin or mechanism. To fulfill VAC criteria, a mechanically ventilated patient must have at least 2 days of stability or improvement of respiratory parameters such as a stable or decreasing daily minimum positive end-expiratory pressure (PEEP) or fraction of inspired oxygen (FiO2) followed by at least 2 days of worsened oxygenation diagnosed by an increase of the daily minimum PEEP (at least 3 cm H2O) or FiO2 (at least 20%). The second criteria IVAC aims to confirm the subgroup of VAC that is potentially related to infection-related complications. VAC associated with an abnormal white blood cell count, altered/modified temperature, with initiation of new antimicrobial agent continued for at least 4 days becomes an IVAC. In addition on or after calendar day 3 of the mechanical ventilator, positive culture from any of the CDC-recommended respiratory samples with significant quantitative or semiquantitative growth, and purulent respiratory secretions with organisms identified from respiratory specimens confirm VAP.
In Phase 2: Unlike Phase 1, interventions include targeted continuous surveillance which aims to monitor high-risk populations and their infection sites. Regular monitoring of all healthcare workers toward their hand hygiene practices, appropriate use of gloves, and insertion/maintenance care bundle approach by trained staff in-charge with checklist got implemented [Chart 1]. Based on the observation, regular educational sessions and workplace monitoring of their compliance were documented. Regular instrumental and environmental cleaning under supervision was insisted upon. For all new staff, regular induction sessions on standard precaution, infection control measures, and ventilator care bundle approach were re-emphasized. Daily auditing, reassessment, and reauditing with feedback discussions were introduced. Based on the culture reports, isolation, and contact precaution policies were strictly implemented.[9-11]

- Ventilator-associated pneumonia prevention bundle care checklist.
Statistical analysis
Audited data was captured in Excel sheets for analysis. A comparison of isolated bacteria and their combination was done using frequency and percentage due to the few number of isolates. Percentage calculation was done for all categorical variables.
RESULTS
From January 2016 to December 2019, a total of 2086 patients were treated in the CCU. A total of 1220 (58.5%) patients got intubated and were provided with mechanical ventilator support, with an average of 3–4 adult patients on ventilator support per day. A total of 5922 ventilator days were recorded out of 1220 critically ill patients on mechanical ventilator support. A total of 49 (4%) patients developed VAP during this 4 years study period [Table 1]. The cumulative incidence rate of VAP was found to be 8.3/1000 ventilator days (Number of patients who developed VAP/total number of ventilator days for the month × 1000).
| Before intervention | After intervention | |||
|---|---|---|---|---|
| 2016 | 2017 | 2018 | 2019 | |
| Total number of patients treated in intensive care unit (n=2086) | 460 | 500 | 596 | 530 |
| Total number of patients on ventilator support (n=1220) | 253 (55%) | 317 (63%) | 313 (53%) | 337 (64%) |
| Total number of Ventilator days | 1494 | 1708 | 1334 | 1386 |
| Total number of patients who developed VAP (n=49) | 16 (6.3%) | 18 (5.6%) | 13 (4.2%) | 2 (0.6%) |
| Incidence of VAP | 10.7 | 10.5 | 9.7 | 1.4 |
VAP: Ventilator-associated pneumonia
During phase 1: (2016–2017) study period, the incidence of VAP was 10.7 and 10.5/1000 ventilator days without interventions. Following intervention and auditing in phase 2 the incidence of VAP was 9.7 VAP/1000 ventilator days in 2018. Further, in the implementation period (2019), the incidence of VAP was significantly reduced to 1.4 VAP/1000 ventilator days. The average ventilator day support for each patient before the onset of VAP was found to be between 5 and 9 days post-intubation [Graph 1].
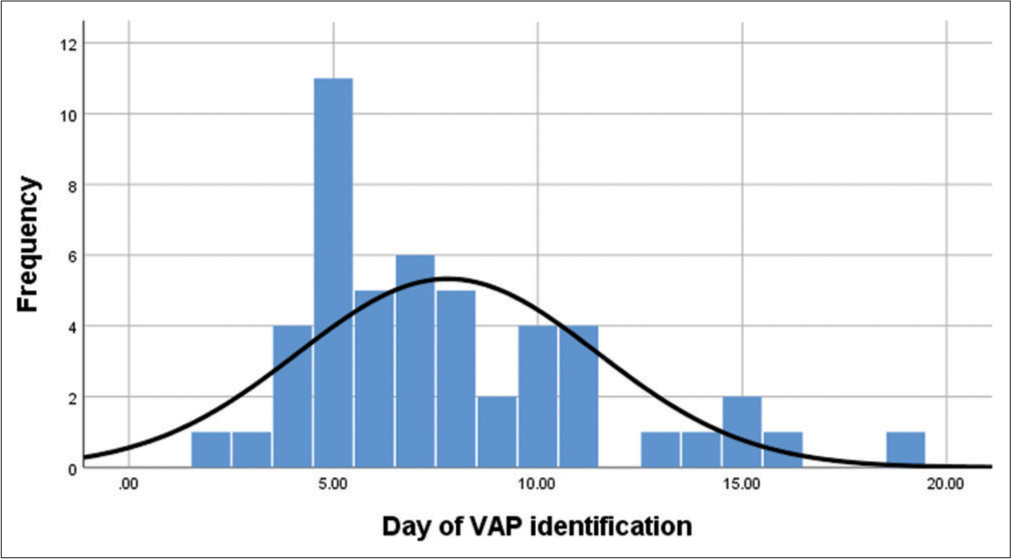
- Frequency distribution of ventilator-associated pneumonia identification day.
Patients betweens 51–66 years were found to be in higher risk groups than other age groups for developing VAP in this study [Table 2]. In addition, the male gender (67%) was found to have higher risk of developing VAP than females (33%). The age group division was done according to the institute’s experience and the flow of patients.
| Age distribution | Before inervention | After intervention | ||||
|---|---|---|---|---|---|---|
| 2016 | 2017 | n=34 (%) | 2018 | 2019 | n=15 (%) | |
| 19–34 years | 3 | 3 | 6 (17.5) | 1 | 0 | 1 (6.6) |
| 35–50 years | 4 | 1 | 5 (14.7) | 2 | 1 | 3 (20) |
| 51–66 years | 7 | 11 | 18 (53) | 3 | 1 | 4 (26.6) |
| ≥ 67 years of age | 2 | 3 | 5 (14.7) | 7 | 0 | 7 (46.6) |
Based on the endotracheal (ET) aspirate culture reports, Gram-negative bacilli (GNB) were found to be the most common pathogen isolated. Among GNB’s, Klebsiella pneumoniae was found to be the most common isolates, followed by Pseudomonas aeruginosa, Acinetobacter baumannii, and others [Table 3].
| Organisms isolated | Before intervention | After intervention | ||||
|---|---|---|---|---|---|---|
| 2016 | 2017 | n=49 (%) | 2018 | 2019 | n=28 (%) | |
| Klebsiella pneumoniae | 3 | 12 | 15 (31) | 6 | 1 | 7 (25) |
| Enterobacter aerogenes | 0 | 2 | 2 (4) | 3 | 0 | 3 (11) |
| Citrobacter freundii | 1 | 0 | 1 (2) | 0 | 0 | 0 |
| Escherichia coli | 0 | 0 | 0 | 1 | 0 | 1 (3.5) |
| Acinetobacter baumannii | 8 | 10 | 18 (37) | 8 | 1 | 9 (32) |
| Pseudomonas aeruginosa | 7 | 4 | 11 (22) | 8 | 0 | 8 (28.5) |
| MSSA | 1 | 0 | 1 (2) | 0 | 0 | 0 |
| MRSA | 1 | 0 | 1 (2) | 0 | 0 | 0 |
The individual bacterial isolates showed a gradual decrease in the trend over the study period, especially in the case of K. pneumoniae, P. aeruginosa, and A. baumannii [Table 3]. Out of a total of 49 culture-positive ET samples, 20 samples grew polymicrobial isolates. K. pneumoniae + P. aeruginosa (35%) was found to be the most common polymicrobial combination among these patients, followed by A. baumannii + P. aeruginosa, Enterobacter aerogenes + K. pneumoniae, E. aerogenes + A. baumannii combination of 10% each, and then others [Table 4]. Following intervention, the trend of polymicrobial isolation rate was found to be gradually decreasing. With the remaining 29 ET samples, the monomicrobial infection rate was documented [Graph 2].
| Before intervention | After intervention | Total | |||
|---|---|---|---|---|---|
| 2016 | 2017 | 2018 | 2019 | ||
| K. pneumoniae+P. aeruginosa | 2 | 3 | 2 | 0 | 7 |
| E. aerogenes+A. baumannii | 0 | 2 | 1 | 0 | 3 |
| A. baumannii+MRSA | 1 | 0 | 0 | 0 | 1 |
| E. aerogenes+P. aeruginosa | 0 | 0 | 1 | 0 | 1 |
| A. baumannii+P. aeruginosa | 1 | 1 | 1 | 0 | 3 |
| K. pneumoniae+A. baumannii | 0 | 1 | 1 | 0 | 2 |
| A. baumannii+MSSA | 1 | 0 | 0 | 0 | 1 |
| E. aerogenes+K. pneumoniae | 0 | 1 | 1 | 0 | 2 |
| Total | 5 | 8 | 7 | 0 | 20 |
K. pneumonia: Klebsiella pneumonia, P. aeruginosa: Pseudomonas aeruginosa, E. aerogenes: Enterobacter aerogenes, A. baumannii: Acinetobacter baumannii, MSSA: Methicillin-susceptible Staphylococcus aureus, MRSA: Methicillin-resistant Staphylococcus aureus
The bacterial isolates in each year were not statistically significant to analyze its resistance pattern individually. Collectively, these isolates showed an increasing trend in its resistance pattern [Graph 2]. The majority of GNB (57%) were found to be multi drug resistant (MDR) isolates. Out of 29 Enterobacterales, 79% (23) were found to be resistant to third-generation cephalosporin and 69% (20) were resistant to fluoroquinolone and cotrimoxazole, followed by 55% (16) resistance to beta-lactam-beta-lactamase inhibitors. A. baumannii showed 100% resistance on ampicillin and ceftriaxone. Moreover, toward gentamicin 78%, amikacin 88%, and on carbapenems and piperacillin-tazobactam 70% resistance was documented followed by others. P. aeruginosa showed 63% resistance on ceftazidime, 36% on ciprofloxacin, 21% toward gentamicin, and others [Graph 3]. During the 4year study period only Staphylococcus aureus (2) got isolated in 2016. This, one was identified as MRSA and it was susceptible to most of the drugs tested. Both the isolates were 100% resistant to penicillin and 50% resistant to gentamicin, and ciprofloxacin, and 100% susceptible to clindamycin, tetracycline, erythromycin, cotrimoxazole, vancomycin, teicoplanin, and linezolid.
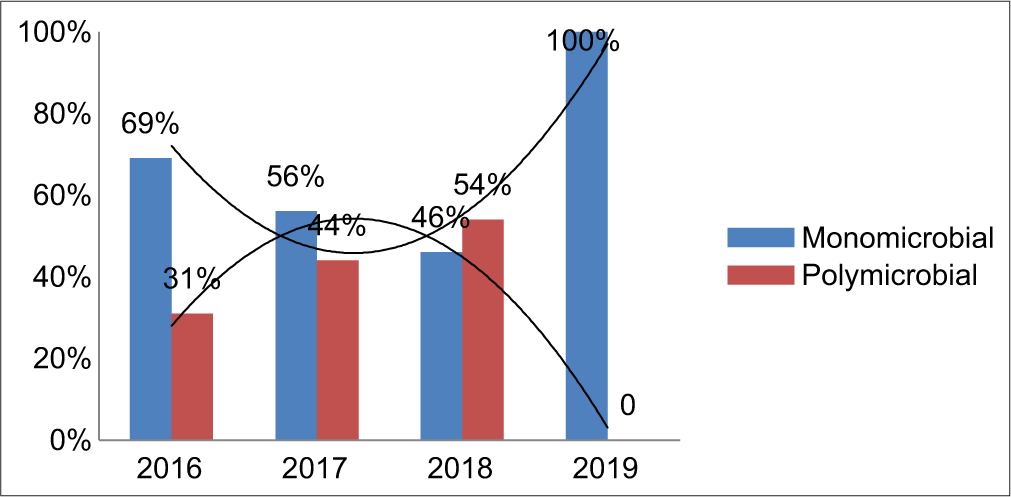
- Incidence of microbial combination.
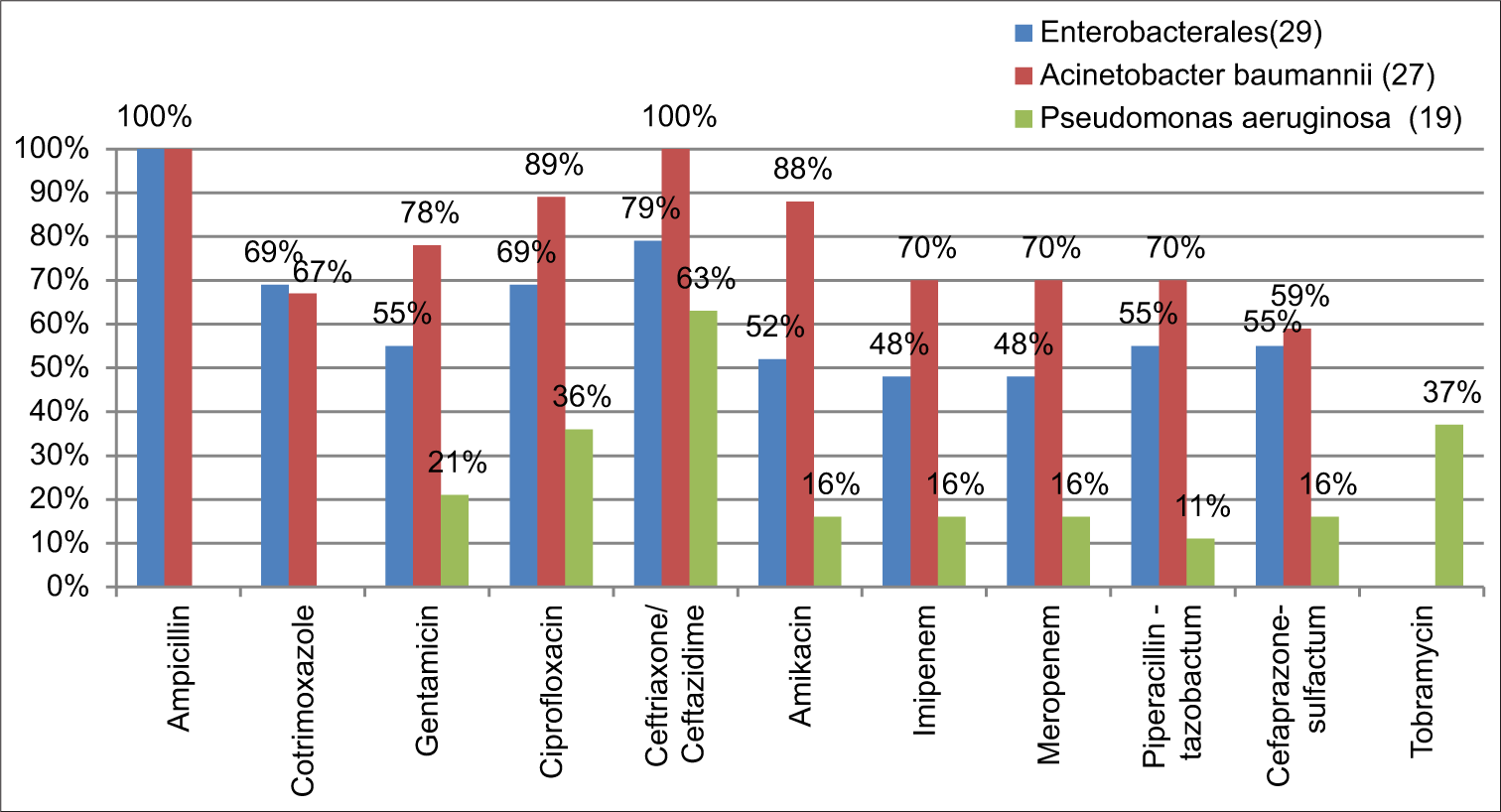
- Antibiotic resistance pattern.
DISCUSSION
Healthcare-acquired infections (HAIs) occur as a result of compromised hand hygiene practices, aseptic medical device insertion technique, prolonged hospitalization, inadequate interventions, and inappropriate antimicrobial usage.[12] According to the WHO, over 1.4 million people worldwide suffer at a time from infectious complications following HAI.[8] Among patients with invasive mechanical ventilation, VAP is one of the most common HAIs. VAP is defined as the infection of the pulmonary parenchyma in patients exposed to invasive mechanical ventilation for a minimum of 48 hours. During the past few decades, especially in developed countries, adequate and effective HAI control programs are being implemented. Instead, due to a lack of awareness and ineffective implementable policies, the incidence of VAP remains peaking in developing countries. In addition, there is no adequate comparative data among developing countries to self-validate the safe health-care delivery system and infection prevention. VAP is one of the leading causes of high mortality among critically ill patients throughout the globe.[13]
In the present performance improvement study, the VAP rate was significantly high in 2016 and 2017. Internal benchmark value was derived using the previous year’s VAP incidence rate. Fixing the internal benchmark value as 6 VAP/1000 ventilator days, in phase 2: various interventions such as continuous active surveillance and infection prevention measures targeting all high dependent areas were introduced. Probable route cause analyses were made for individual VAP patients. Corrective and preventive measures such as regular educational sessions for all healthcare workers on VAP prevention, care bundle monitoring, contact precaution, and regular hand hygiene practices played a pivotal role in bringing the VAP incidence to 1.4 VAP/1000 ventilator days during 2019 [Table 1]. Very similar to this, according to the International Nosocomial Infection Control Consortium, CDC report, between 2007 and 2012 involving 43 countries in a device-associated module, the overall rate of VAP/1000 mechanical ventilator days was 14.7 and 9.54 in adult and pediatric intensive care units, repectively.[14,15] The pooled VAP data of 40 hospitals from 20 cities in India (10 years of data) state that the incidence was 9.4.[16] The CDC NHSN data of 2013 state a VAP rate of 2.0/1000 device days.[14] In contrast, a recent study from China documented (2012–2019) 22.68 VAP/1000 device days.[17] Similarly, a study by Deepashree et al., from Puducherry, India during 2015-2016, documented a VAP rate of 25/1000 device days.[18]
In general, based on the duration, the early-onset VAP (less than the initial 5 days of hospitalization and intubation) and late on-set VAP (after 5 days of hospitalization and intubation) clinical diagnosis will be made.[19] A recent Cochrane review (2023) reported a high incidence of late-onset VAP.[20] Similarly, the majority of the VAP documented during these 4 years were found to be late-onset VAP (>96 h). With increasing age between 51 and 66 years, the incidence of VAP was found to be significant along with male gender predominance.[17]
Among the pathogens, gram-negative organisms were found to be the common cause of VAP followed by Gram-positive organisms.[17,21,22] Similar to this present study, a study by Gragueb-Chatti et al., in 2021, documented K. pneumoniae as the most common pathogen and among all the nonfermenter, A. baumanniiand, P. aeruginosa, was found to be the predominant pathogen.[23] In contrast, a recent study from India and China (2023) documents, A. baumannii as the most common pathogen to develop VAP, followed by K. pneumoniae and P. aeruginosa.[17,24] Similar to other studies, 79% of Enterobacterales and 100% of A. baumannii were found to be resistant to third-generation cephalosporin.[25] About 48% of Enterobacterales, 70% of A. baumannii, and 16% of P. aeruginosa were found to be Carbapenemase producers. Similar to the present study, few studies says that MDR isolates of P. aeruginosa are increasingly prevalent among VAP patients and also reported that one-half to two-thirds of A. baumannii strains causing VAP are currently found to be Carbapenem-resistant.[26] These isolates were also found to carry blaOXA-23 encoding genes followed by blaOXA-66 in India.[24] The incidence of MRSA in causing VAP is in the decline phase and ths might be due to stringent infection control and preventive measures.
Fortunately, over a period of time due to multidimensional approaches such as close monitoring, strict hand hygiene practices, staff training, assessment, feedback review, proper use of PPEs, and care bundle approach, the trend of organisms showed a decline (bell-shaped curve) pattern. During 2019, when compared to previous years, the microbial combination also changed from polymicrobial to monomicrobial cause. This could be due to improved environmental disinfection processes, barrier nursing, isolation precautions, and other infection control measures with the care bundle approach [Chart 1].[26,27]
CONCLUSION
The incidence of late-onset VAP was found to be higher than early-onset VAP. Males aged 51–66 were found to be in higher risk groups in this setup. Gram negatives, especially K. pneumoniae, P. aeruginosa, and A. baumannii were found to be the most common bacterial pathogenss with the significant resistant pattern. The duration of the mechanical ventilation needs to be reduced by administering proper weaning protocol and titrating the sedation regimens. With proper nursing care, strict hand hygiene, barrier nursing, antibiotic policy, precise diagnostic tools, proactive surveillance networking, and VAP care bundle approach, the burden of VAP can be kept within the benchmark value depending on the individual healthcare setup.
Acknowledgment
Our sincere thanks to Sri Balaji Vidyapeeth Deemed-to-be University for supporting this faculty project. We thank all our Infection Control Nurses, Critical Care Medicine team, Nursing team, Statistician, and Housekeeping team members for their valuable contribution, cooperation, and support.
Ethical approval
The author(s) declare that they have taken the ethical approval from IRB/IEC.
Declaration of patient consent
Patient consent not required as there are no patients in this study.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The author(s) confirms that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Health Care-associated Infections-an Overview. Infect Drug Resist. 2018;11:2321-33.
- [CrossRef] [PubMed] [Google Scholar]
- Ventilator-associated Pneumonia in Adults: A Narrative Review. Intensive Care Med. 2020;46:888-906.
- [CrossRef] [PubMed] [Google Scholar]
- Ventilator-associated Pneumonia in Adult Intensive Care Unit Prevalence and Complications. Egypt J Crit Care Med. 2017;5:61-3.
- [CrossRef] [Google Scholar]
- Practical Strategies to Reduce Nosocomial Transmission to Healthcare Professionals Providing Respiratory Care to Patients with COVID-19. Crit Care. 2020;24:571.
- [CrossRef] [PubMed] [Google Scholar]
- Management of Adults with Hospital-acquired and Ventilator-associated Pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63:e61-111.
- [CrossRef] [PubMed] [Google Scholar]
- Ventilator-associated Pneumonia. Intensive Care Med. 2022;48:1222-6.
- [CrossRef] [PubMed] [Google Scholar]
- Risk Factors of Ventilator-associated Pneumonia in Critically III Patients. Front Pharmacol. 2019;10:482.
- [CrossRef] [PubMed] [Google Scholar]
- Device Associate Module: Ventilator-Associated Event (VAE) Atlanta, U S A: Centers for Disease Control and Prevention; 2016. p. :1-46.
- [Google Scholar]
- Infection Control in the Intensive Care Unit: Expert Consensus Statements for SARS-CoV-2 Using a Delphi Method. Lancet Infect Dis. 2022;22:e74-87.
- [CrossRef] [PubMed] [Google Scholar]
- Risk Factors for Ventilator-associated Pneumonia in Trauma Patients: A Descriptive Analysis. World J Emerg Med. 2018;9:203-10.
- [CrossRef] [PubMed] [Google Scholar]
- Developing a new, national approach to surveillance for ventilator-associated events: Executive summary. Clin Infect Dis. 2013;57:1742-6.
- [CrossRef] [PubMed] [Google Scholar]
- World Alliance for Patient Safety. The Global Patient Safety Challenge 2005-2006 “Clean Care is Safer Care”. 2005. Geneva, Switzerland: WHO; Available from: https://www.who.int/gpsc/en [Last accessed on 2008 Dec 01]
- [Google Scholar]
- Estimating the Burden of Antimicrobial Resistance: A Systematic Literature Review. Antimicrob Resist Infect Control. 2018;7:58.
- [CrossRef] [PubMed] [Google Scholar]
- International Nosocomial Infection Control Consortium (INICC) Report, Data Summary of 43 Countries for 2007-2012. Device-associated Module. Am J Infect Control. 2014;42:942-56.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical Epidemiology and Outcomes of Ventilator-associated Pneumonia in Critically Ill Adult Patients: Protocol for a Large-scale Systematic Review and Planned Meta-analysis. Syst Rev. 2019;8:180.
- [CrossRef] [PubMed] [Google Scholar]
- Device-associated Infection Rates in 20 Cities of India, Data Summary for 2004-2013: Findings of the International Nosocomial Infection Control Consortium. Infect Control Hosp Epidemiol. 2016;37:172-81.
- [CrossRef] [PubMed] [Google Scholar]
- A Prospective Surveillance Study of Healthcare-associated Infections in an Intensive Care Unit from a Tertiary Care Teaching Hospital from 2012-2019. Medicine (Baltimore). 2023;102:e34469.
- [CrossRef] [PubMed] [Google Scholar]
- Implementation of Active Surveillance System to Track Hospital-acquired Infections in a Tertiary Care Hospital in India. J Curr Res Sci Med. 2017;3:21-8.
- [CrossRef] [Google Scholar]
- Hospital-acquired and Ventilator-associated Pneumonia: Diagnosis, Management, and Prevention. Cleve Clin J Med. 2020;87:633-9.
- [CrossRef] [PubMed] [Google Scholar]
- Length of Intensive Care Unit Stay and the Apparent Efficacy of Antimicrobial-based Versus Non-antimicrobial-based Ventilator Pneumonia Prevention Interventions within the Cochrane Review Database. J Hosp Infect. 2023;140:46-53.
- [CrossRef] [PubMed] [Google Scholar]
- Keeping it Simple: Impact of a Restrictive Antibiotic Policy for Ventilator-associated Pneumonia in Trauma Patients on Incidence and Sensitivities of Causative Pathogens. Surg Infect (Larchmt). 2018;19:672-8.
- [CrossRef] [PubMed] [Google Scholar]
- Resistance Trends and Treatment Options in Gram-negative Ventilator-associated Pneumonia. Curr Infect Dis Rep. 2018;20:3.
- [CrossRef] [PubMed] [Google Scholar]
- Systematic Review of Studies Investigating Ventilator Associated Pneumonia Diagnostics in Intensive Care. BMC Pulm Med. 2021;21:196.
- [CrossRef] [PubMed] [Google Scholar]
- Burden of Carbapenem-resistant Acinetobacter baumannii Harboring blaOXA Genes in the Indian Intensive Care Unit. Glob J Med Pharm Biomed Update. 2023;18:12.
- [CrossRef] [Google Scholar]
- Health-care-associated Infections: Risk Factors and Epidemiology from an Intensive Care Unit in Northern India. Indian J Anaesth. 2014;58:30-5.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence and Etiology of Community-and Hospital-acquired Pneumonia in Saudi Arabia and their Antimicrobial Susceptibility Patterns: A Systematic Review. Medicina (Kaunas). 2023;59:760.
- [CrossRef] [PubMed] [Google Scholar]
- The Impact of a Ventilator Bundle on Preventing Ventilator-associated Pneumonia: A Multicenter Study. Am J Infect Control. 2014;42:34-7.
- [CrossRef] [PubMed] [Google Scholar]







