Translate this page into:
Glucose and Insulin Activities in the Leaf Extracts of Aloe vera, Bryophyllum, and Ivy Gourd

*Corresponding author: Sabitha Kandi, Department of Biochemistry, Prathima Institute of Medical Sciences, Karimnagar, Telangana, India. tejaswani19@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Kandi S, Kollu R, Boddula V, Kandi V. Glucose and insulin activities in the leaf extracts of Aloe vera, Bryophyllum, and Ivy gourd. Glob J Med Pharm Biomed Update 2023;18:15.
Abstract
Objectives:
Allopathic medicines, although they play a crucial role in controlling blood sugars among diabetic patients, alone may be insufficient for the effective management of diabetes. Therefore, it is essential to explore the food for its anti-diabetic potential and delay the development of long-term complications of this debilitating disease. Aloe vera, Bryophyllum, and Ivy gourd are edible and, if included in daily food, could contribute to preventing and managing diabetes. In this study, we have estimated the glucose and insulin concentrations of A. vera, Bryophyllum, and Ivy gourd plant extracts.
Materials and Methods:
The leaf extracts of A. vera, Bryophyllum, and Ivy gourd were assessed for glucose and insulin. Glucose was estimated using the glucose-oxidase peroxidase method and insulin was measured using the enzyme-linked immunosorbent assay.
Results:
Ivy gourd leaf extract revealed the highest concentration of both glucose and insulin at concentrations of 56 mg/dL and 46.46 µIU/mL, respectively. Bryophyllum leaf extracts revealed moderate concentrations of insulin (24.14 µIU/mL) and glucose (23.11 mg/dL). Among the extracts tested, the A. vera extract revealed the lowest concentrations of glucose (22 mg/dL) and insulin (10.87 µIU/mL).
Conclusions:
A. vera, Bryophyllum, and Ivy gourd leaves have reasonable concentrations of insulin which could be explored for pharmacological purposes. Moreover, being edible, these could be included in the diet as alternative methods to prevent and manage diabetes.
Keywords
Aloe vera
Bryophyllum
Ivy gourd
Diabetes
Plant extracts
Anti-diabetic
Edible
INTRODUCTION
Medicinal plants synthesize phytochemicals that help their defense against parasites such as insects, fungi, other microbial diseases, and herbivorous mammals.[1] The phytochemicals contain plant-based essential components which could become a source of nutrients to humans and, therefore, included in the diet.[2] Phytochemicals are being used as supplements to manage diseases.[3] By composition, phytochemicals may be alkaloids, glycosides, polyphenols, and terpenes among others.[4] However, these may be toxic (phytotoxin) and carcinogenic in nature.[5] Most phytotoxin-containing plant material is avoided by humans or processed to eliminate the toxins before they are consumed as food.[6] The phytochemicals are thermolabile and, hence, are lost in cooking and food processing techniques.[7] The phytochemical constituents of plants help in lowering blood glucose levels by regulating glucose and also could delay associated complications.[8] Diabetes is the most prevalent non-communicable disease and billions of people are affected by it throughout the world.[9] There are several causes for the development of diabetes. The predisposing factors for diabetes include sedentary lifestyles, lack of physical activity, consuming a high-sugar and fat diet, insulin resistance, and inadequate secretion of insulin by the pancreas among others. Moreover, genetic predisposition is also an established factor for diabetes. Furthermore, persons with diabetes are prone to long-term health complications and suffer from uncontrolled diabetes despite treatment.[10] Therefore, it is essential to explore alternative methods in the form of naturally available and edible resources like plants for their role in controlling and preventing diabetes.
Aloe vera (Aloe barbadensis miller) is a shrubby, perennial, succulent, xerophytic, and perennial plant belonging to the Liliaceae family. It grows in topical, sub-tropical, and arid regions of the world. It has been known for more than 2000 years and is popular as a plant with medicinal properties.[11] The Bryophyllum (Kalanchoë) plants belong to the family Crassulaceae and are found throughout the world. Bryophyllum species have been identified as plants with potential medicinal properties that can be used as complementary/alternative medicine.[12] Ivy gourd is scientifically called Coccinia grandis and belongs to the Cucurbitaceae family. It grows in tropical regions of the world including India where it is consumed as a vegetable. The leaf extract and fruit of C. grandis have been explored for their medicinal properties.[13]
The present study is carried out to assess the glucose and insulin concentrations in the leaf extracts of A. vera, Bryophyllum, and Ivy Gourd.
MATERIAL AND METHODS
The leaves of A. vera, Bryophyllum, and Ivy gourd were collected from the households [Figure 1].

- Leaf extract, (a): Aloe vera, (b): Ivy gourd, (c): Bryophyllum.
The leaves were carefully cleaned to remove the dust before being processed. The leaves were then ground using mortar and pestle and by adding little quantities of water as needed. The extract was filtered using filter paper and the filtrate was used for the quantitative measurements of glucose and Insulin.
The glucose levels in the filtrate obtained from leaf extract were measured by the glucose-oxidase peroxidase (GODPOD) method in a colorimeter. The insulin concentrations were measured using Enzyme-Linked Immunosorbent Assay using Qayee – bio kit for life science.
The potential mechanism of the action and effects of the A. vera, Bryophyllum, and ivy gourd extracts on the GOD-POD assay are shown in [Figure 2].
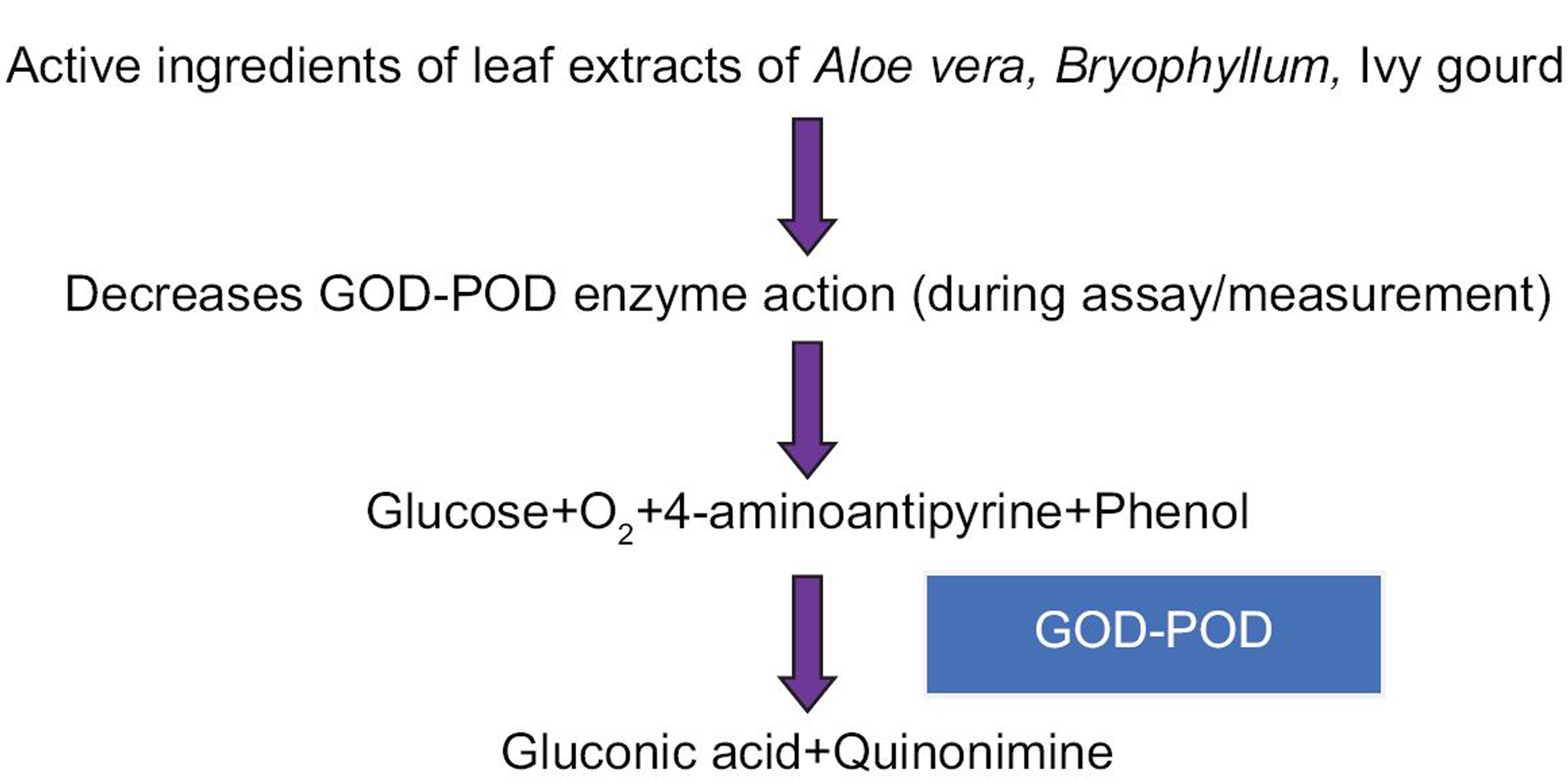
- Flow chart depicting action of active ingredients of leaf extract on glucose-oxidase peroxidase assay.
Procedure for measuring the insulin concentrations
In a microwell, 40 µL of diluent is mixed with 10 µL of leaf extract. Similarly, 50 µL of standard reagent was added into another well that is used for standard reading. A 50 µL of horse radish peroxidase was added to both wells. The plate was, then, sealed with aluminum foil. The microwell plate was carefully shaken and incubated at 37°C for 60 min. The contents in the microwells were discarded and a diluted wash liquid was added to the wells. The microwell plate was shaken and the contents were discarded. The microwells were tapped on an absorbent paper and allowed to dry completely. This process was repeated 5 times. Later, 50 µL of chromogen A was added to each well followed by the addition of chromogen B. The microwell plate was shaken and incubated at 37°C for 10 min away from light. A 50 µL stop solution was added to each well and the optical density values were measured at 450 nm.
RESULTS
Ivy gourd leaf extract revealed the highest concentration of both glucose and insulin at concentrations of 56 mg/dL and 46.46 µIU/mL, respectively. Bryophyllum leaf extracts revealed moderate concentrations of insulin (24.14 µIU/ mL) and glucose (23.11 mg/dL). Among the extracts tested, the A. vera extract revealed the lowest concentrations of glucose (22 mg/dL) and insulin (10.87 µIU/mL). The glucose and insulin concentrations observed in the plant extracts of A. vera, Bryophyllum, and Ivy gourd are shown in [Table 1].
| Source of extract | Glucose levels | Insulin levels |
|---|---|---|
| Aloe vera | 22 mg/dL | 10.87 µIU/mL |
| Bryophyllum | 23.11 mg/dL | 24.14 µIU/mL |
| Ivy gourd | 56 mg/dL | 46.46 µIU/mL |
The bar diagram showing glucose and insulin concentrations in the leaf extracts concerning the absorbance is shown in [Figures 3 and 4], respectively.

- The glucose concentrations of leaf extract concerning absorbance.

- The insulin concentrations of leaf extract concerning absorbance.
DISCUSSION
Diabetes is a non-communicable disease that previously was commonly noticed among older persons. However, in recent times, diabetes has been a frequent cause of concern among young adults and middle-aged persons. This could be attributed to the changing food habits and dormant/ sedentary lifestyles. Uncontrolled diabetes can lead to several complications that include cardiovascular and kidney diseases, among others.
The constituents of plant phytochemicals and their benefits to human health have been adequately established. Alkaloids are natural organic compounds that possess at least one nitrogen atom. They are bitter and are products of nitrogen metabolism in plants. Alkaloids (synthetic/semi-synthetic) are used as drugs to enhance the primary effects of drugs and to reduce unwanted side effects.[14] The medically important alkaloids and their potential applications are depicted in [Table 2].
| Alkaloid | Application |
|---|---|
| Morphine | Analgesic |
| Nicotine | Stimulant, and nicotine-acetyl choline receptor agonist |
| Reserpine | Antihypertensive |
| Vinblastine | Anti-tumor |
| Quinine | Antipyretic and antimalarial |
Glycosides are phytochemical compounds that are made up of sugars that are interconnected by glycoside bonds. Plants store chemicals in the form of inactive glycosides which are activated by hydrolysis. Many glycosides are used in medications.[15] Plant glycosides and their medical applications are shown in [Table 3].
| Glycosides | Application |
|---|---|
| Alcoholic glycosides | Analgesic, antipyretic |
| Phenolic glycosides | Anti-inflammatory effects, urinary antiseptic effects |
| Flavonoid glycosides | Antioxidant effects, decreases capillary fragility |
| Coumarin glycosides | Blocks calcium channels, dilates coronary arteries |
| Cardiac glycosides | Treatment of cardiac diseases |
Polyphenols are organic compounds that contain many phenol units (C6H5OH). These are abundantly present in plants. Polyphenols are structurally diverse and can be further classified as flavonoids (present in apples, onions, etc.), phenolic acids (fruits, vegetables, etc.), polyphenolic amides (pepper, oats, etc.), and other polyphenols (sesame seeds, flax seeds, red wine, etc.).[15,16] Polyphenols help in protection against ionizing radiation and microbial infections, maintain the green pigment in plants, and regulate the growth hormone during the fruit ripening process.[17,18] Polyphenols contribute to normal vascular endothelial function and also protect blood lipids from oxidative damage in humans.[19,20]
Terpenes are a group of unsaturated hydrocarbons produced by plants, especially conifers.[21] They are mediators of ecological interactions and help plants in their defense against herbivores and diseases. They are major biosynthetic building blocks that include steroids, which are derivatives of triterpenes. Terpenes are the primary constituent of the essential oil of plants and their flowers.[22] They facilitate the growth and elongation of plants, membrane permeability, and fluidity control.[23] Terpenes act as natural rubber (polymer of isoprene) and have commercial value. They are used as constituents of ink, varnishes, and adhesives, and as fragrances in perfumes, cosmetics, and cleaning products.[24,25] Terpenes are used as components of traditional medicine such as aromatherapy.[26] They are also used as pesticides in agriculture.[27]
Some plants produce active ingredients known as saponins (pseudo prototinosaponin, prototinosaponin). The saponins act on glucose uptake as well as insulin release affecting hepatic gluconeogenesis or glycogenolysis.[28] It was experimentally proved in diabetic rats that bitter content aloin, a saponin of A. vera has a hypoglycemic effect.[29] In addition, the beneficial effects of A. vera in the management of diabetes were explored both in vitro and by animal experiments in a previous study by AboYoussef and Messiha.[30] The results of this study had clearly indicated the efficacy of A. vera extracts over the traditional drug (glimiperide) in the control of blood sugar (93.66 ± 26.92 mg/dL vs. 117.43 ± 21.96 mg/dL) and in the regulation of insulin activities (6.78 ± 0.98 ng/mL vs. 6.26 ± 0.65 ng/ mL). The saponins present in medicinal plants are known for their hypolipidemic, hypoglycemic, and anti-cancerous agents.[31] The drugs used for various health issues are manufactured using saponin contents.
The saponins are amphipathic plant products with diverse functions. The attachment of sugar to sterol or triterpenes makes saponin amphipathic and is also the reason for forming a gel-like or soapy substance. Saponins are used both in conventional and traditional medicine.[32-34]
Bryophyllum is known to possess bufadienolide, which is a glycoside that confers various health benefits.[35,36] It exerts secretagogue (a substance that causes secretion of another substance) action on beta-cells of the pancreas by the closure of potassium-adenosine triphosphate channels which stimulate the release of insulin similar to sulfonylurea.[37]
The Ivy gourd is a plant with anti-anaphylactic and antihistaminic properties and is used as a traditional medicine. The active constituents are taraxerone and taxero (triterpenoid), amyran, and lupeol.[38] The compounds present in Ivy gourd are noted to regulate blood glucose levels by inhibiting the glucose-6-phosphatase enzyme.[39]
The coordinated activity of the liver and muscle helps in glucose homeostasis. In postprandial states, the liver helps in glycogenesis and leads to glycogenolysis in the fasting state.[40] The imbalance between the uptake of glucose in muscle and the overproduction of glucose in the liver creates insulin sensitivity.[41] The saponins stimulate AKT/ protein kinase B signaling pathway, leading to hypoglycemic effects. The AKT improves glucose uptake of skeletal muscle by activating Glucose Transporter 4.[42] The probable mechanism by which the A. vera, Bryophyllum, and Ivy gourd plant extracts have on the management and control of blood glucose and insulin activities is depicted in [Figure 5].
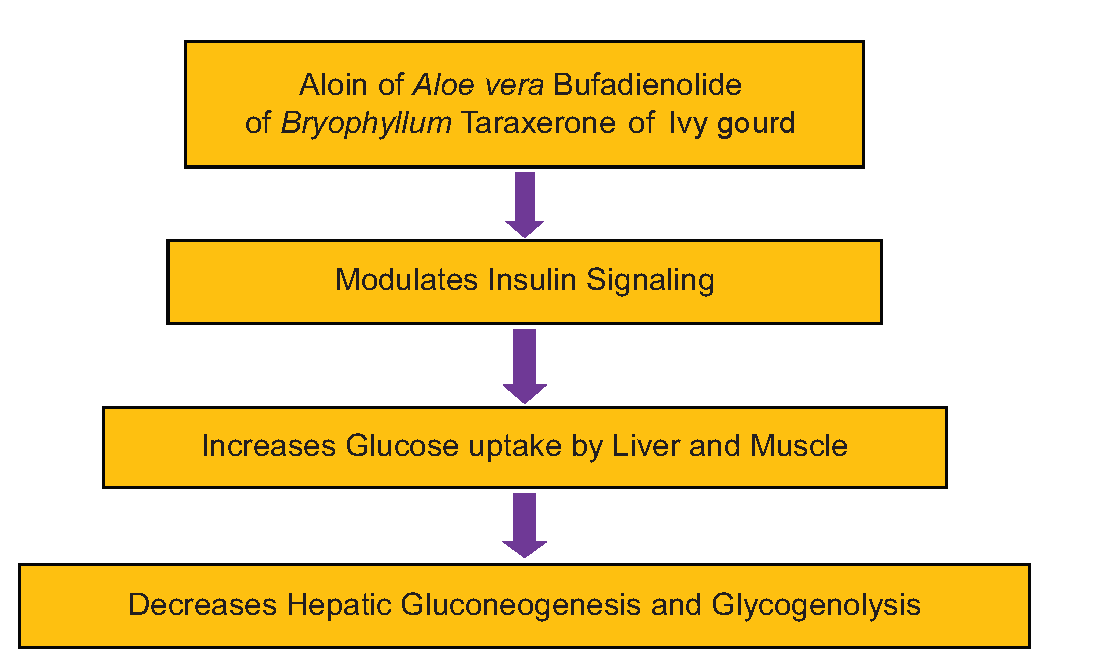
- Flow chart depicting the probable mechanism of action of leaf extract active ingredients on insulin activities and blood glucose concentrations.
CONCLUSION
A. vera, Bryophyllum, and Ivy gourd leaf extracts have reasonable concentrations of insulin which could be explored for pharmacological purposes. The active ingredients including saponins, alkaloids, glycosides, polyphenols, and terpenes present in these plants could be investigated for their pharmacological potential and medicinal properties. Moreover, being edible, these could be included in the diet as alternative methods to prevent and manage diabetes and its related long-term complications.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Conflicts of interest
There are no conflicts of interest.
Financial support and sponsorship
Nil.
References
- Animal self-medication and ethno-medicine: Exploration and exploitation of the medicinal properties of plants. Proc Nutr Soc. 2003;62:371-81.
- [CrossRef] [PubMed] [Google Scholar]
- Diet, nutrients and the microbiome. Prog Mol Biol Transl Sci. 2020;171:237-63.
- [CrossRef] [PubMed] [Google Scholar]
- Medicinal plants, phytochemicals, and herbs to combat viral pathogens including SARS-CoV-2. Molecules. 2021;26:1775.
- [CrossRef] [PubMed] [Google Scholar]
- Potential roles and molecular mechanisms of phytochemicals against cancer. Food Funct. 2022;13:9208-25.
- [CrossRef] [PubMed] [Google Scholar]
- Incorporation of absorption and metabolism into liver toxicity prediction for phytochemicals: A tiered in silico QSAR approach. Food Chem Toxicol. 2018;118:409-15.
- [CrossRef] [PubMed] [Google Scholar]
- Wonder or evil?: Multifaceted health hazards and health benefits of Cannabis sativa and its phytochemicals. Saudi J Biol Sci. 2021;28:7290-313.
- [CrossRef] [PubMed] [Google Scholar]
- Agrifood by-products as a source of phytochemical compounds In: Díaz AV, García-Gimeno RM, eds. Descriptive Food Science. London: IntechOpen; 2018. Available from: https://www.intechopen.com/chapters/63207 [Last accessed on 2022 Nov 15]
- [CrossRef] [PubMed] [Google Scholar]
- Antidiabetic phytochemicals from medicinal plants: Prospective candidates for new drug discovery and development. Front Endocrinol (Lasusanne). 2022;13:800714.
- [CrossRef] [PubMed] [Google Scholar]
- Serum activities of ferritin among controlled and uncontrolled Type 2 diabetes mellitus patients. Cureus. 2022;14:e25155.
- [CrossRef] [Google Scholar]
- Bryophyllum pinnatum and related species used in anthroposophic medicine: Constituents, pharmacological activities, and clinical efficacy. Planta Med. 2016;82:930-41.
- [CrossRef] [PubMed] [Google Scholar]
- The leaf extract of Coccinia grandis (L.) voigt accelerated in vitro wound healing by reducing oxidative stress injury. Oxid Med Cell Longev. 2021;2021:3963510.
- [CrossRef] [PubMed] [Google Scholar]
- Alkaloids used as medicines: Structural phytochemistry meets biodiversity-an update and forward look. Molecules. 2021;26:1836.
- [CrossRef] [PubMed] [Google Scholar]
- "Alkaloid" Wikipedia, Wikimedia Foundation. 2023. Available from: https://wikipedia.org/wiki/alkaloid [Last accessed on 2023 Aug 03]
- [Google Scholar]
- Chemistry and biochemistry of dietary polyphenols. Nutrients. 2010;2:1231-46.
- [CrossRef] [PubMed] [Google Scholar]
- Natural polyphenols for prevention and treatment of cancer. Nutrients. 2016;8:515.
- [CrossRef] [Google Scholar]
- Polyphenols: Food sources and bioavailability. Am J Clin Nutr. 2004;79:727-47.
- [CrossRef] [PubMed] [Google Scholar]
- Influence of polyphenols on baceterial biofilm formation and quorum sensing. Zeitschrift Naturforschung C. 2003;58:879-84.
- [CrossRef] [PubMed] [Google Scholar]
- Scientific opinion on the modification of the authorisation of a health claim related to cocoa flavanols and maintenance of normal endothelium-dependent vasodilation pursuant to article13(5) of regulation (EC)No. 1924/2006 following a request in accordance with article 19 of regulation (EC) No. 1924/2006. EFSA J. 2014;12:3654.
- [CrossRef] [Google Scholar]
- Scientific opinion on the substantiation of health claims related to polyphenols in olive and protection of LDL particles from oxidative damage (ID 1333,1638,1639,1696,2865), maintenance of normal blood HDL cholesterol concentrations (ID 1639) mainte. EFSA J. 2011;9:2033.
- [CrossRef] [Google Scholar]
- Quantitative analysis of bioactive compounds from aromatic plants by means of dynamic headspace extraction and multiple headspace extraction-gas chromotagraphy mass spectrometry. Quantitative analysis of bioactive compounds. J Food Sci. 2016;81:C867-73.
- [CrossRef] [PubMed] [Google Scholar]
- Production and engineering of terpenoids in plant cell culture. Nat Chem Biol. 2007;3:387-95.
- [CrossRef] [PubMed] [Google Scholar]
- Terpenes: Major sources, properties and applications In: Monomers Polymers and Composites from Renewable Resources. Netherlands: Elsevier; 2008. p. :17-38.
- [CrossRef] [Google Scholar]
- The effects of essential oils and terpenes in relation to their routes of intake and application. Int J Mol Sci. 2020;21:1558.
- [CrossRef] [PubMed] [Google Scholar]
- Plant essential oils for pest and disease management. Crop Protect. 2000;21:603-8.
- [CrossRef] [Google Scholar]
- Medicinal plants with potential antidiabetic activity-a review of ten years of herbal medicine research (1990-2000) Int J Diabetes Metab. 2006;14:1-25.
- [CrossRef] [Google Scholar]
- Indian herbs and herbal drugs used for the treatment of diabetes. J Clin Biochem Nutr. 2007;40:163-73.
- [CrossRef] [PubMed] [Google Scholar]
- Beneficial effects of Aloe vera in treatment of diabetes: Comparative in vivo and in vitro studies. Bull Faculty Pharm Cairo Univ. 2013;51:7-11.
- [CrossRef] [Google Scholar]
- Saponins, classification and occurrence in the plant kingdom. Phytochemistry. 2007;68:275-97.
- [CrossRef] [PubMed] [Google Scholar]
- Saponin-based surfactants In: Kjellin M, Johansson I, eds. Surfactants from Renewable Sources Resources. Chichester, UK: John Wiley & Sons Ltd.; 2010. p. :239-49.
- [CrossRef] [Google Scholar]
- Foamy matters: An update on Quillaja saponins and their use as immunoadjuvants. Future Med Chem. 2019;11:1485-99.
- [CrossRef] [PubMed] [Google Scholar]
- Plants of the World Online. Facilitated by the Royal Botanic Gardens, Kew. 2022. Available from: https://www.plantsoftheworldonline.org [Last accessed on 2022 Nov 15]
- [Google Scholar]
- Bufadienolide In: Wikipedia. The Free Encyclopedia; 2021. Available from: https://www.en.wikipedia.org/w/index.php?title=bufadienolide&oldid=1043577025 [Last accessed on 2022 Aug 19]
- [Google Scholar]
- Antidiabetic activity of Kalanchoe pinnata in streptozotocin-induced diabetic rats by glucose independent insulin secretagogue action. Pharm Biol. 2013;51:1411-8.
- [CrossRef] [PubMed] [Google Scholar]
- Platyconic acid, a saponin from Platycodi radix, improves glucose homeostasis by enhancing insulin sensitivity in vitro and in vivo. Eur J Nutr. 2012;51:529-40.
- [CrossRef] [PubMed] [Google Scholar]
- Anti-hyperglycemic and hypolipidemic effects of Cistanche tubulosa in Type 2 diabetic db/db mice. J Ethnopharmacol. 2013;150:935-45.
- [CrossRef] [PubMed] [Google Scholar]
- AMPK activation by prolonged stimulation with interleukin-1β contributes to the promotion of GLUT4 translocation in skeletal muscle cells. Cell Biol Int. 2016;40:1204-11.
- [CrossRef] [PubMed] [Google Scholar]







