Translate this page into:
Cost Analysis of Mirabegron Versus Solifenacin in Overactive Bladder Syndrome

*Corresponding author: Dhanya Sasidharan Palappallil, Department of Pharmacology, Government Medical College, Kottayam, Kerala, India. drspdhanya@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Cyriac T, Raj MO, Palappallil DS, S Syam, Paul F, Kizhedath S. Cost Analysis of Mirabegron Versus Solifenacin in Overactive Bladder Syndrome. Glob J Med Pharm Biomed Update.2025;20:9. doi: 10.25259/GJMPBU_18_2025
Abstract
Objectives:
Overactive bladder (OAB) is a common condition affecting nearly 30% of men and over 50% of women in Asia It is characterized by urinary urgency, frequency, and incontinence. The objective of this study was to determine the cost analysis of mirabegron and solifenacin for the treatment of OAB within the Indian healthcare context.
Material and Methods:
This pharmacoeconomic evaluation was done from the patient’s perspective. The data were obtained from 298 patients (149 each in mirabegron and solifenacin group) over a 90–day treatment period. Effectiveness was assessed using the OAB-Validated-8-question score, while costs were calculated from direct drug expenditures and projected over a 10-year horizon.
Results:
The analysis revealed that mirabegron achieved a greater reduction in OAB symptoms (80.13%) compared to solifenacin (71.32%), with a lower average cost-effectiveness ratio (Rs 3999.74 vs. Rs 7144). Adjusted cost projections for 2031 indicated both drugs would experience price increases, but mirabegron maintained a cost advantage. Furthermore, mirabegron was better tolerated, with no significant adverse drug reactions, whereas mild adverse effects such as dry mouth and constipation were reported in the solifenacin group.
Conclusion:
The study concludes that mirabegron is a superior option in terms of both cost and efficacy for OAB treatment, making it a preferable choice in resource-limited settings. Future research should focus on long-term evaluations incorporating indirect and societal costs to comprehensively assess the economic impact of these treatments.
Keywords
Mirabegron
Overactive bladder
Pharmacoeconomic evaluation
Solifenacin
INTRODUCTION
Overactive bladder (OAB) is a condition characterized by urinary urgency, frequency, and incontinence. The International Continence Society defines OAB as “Urinary urgency, usually with urinary frequency and nocturia, with or without urgency urinary incontinence (UUI).”[1] In the Asian population, the prevalence of OAB is 29.9% in men and 53.1% in women.[2] Along with the mention of urgency in the definition, literature says that there is 20–40% prevalence of incontinence in either gender.[1] Despite the interference with daily activities, there is hesitance in seeking medical help for the symptoms. The management of OAB includes non-pharmacological interventions such as bladder training, pelvic floor muscle training, and lifestyle modifications.
The pharmacological interventions include antimuscarinic medications which relax the bladder muscle such as oxybutynin, tolterodine, solifenacin, fesoterodine, and darifenacin as well as well as Beta-3 adrenergic agonists like mirabegron which relaxes the bladder muscle by stimulating beta-3 receptors.[3]
Mirabegron is considered more effective as compared to solifenacin in the treatment of OAB as assessed by OAB-Validated-8-question (OAB-V-8) awareness tool and it is also associated with a lower risk of anticholinergic side effects compared to solifenacin.[4,5] However, the cost-effectiveness of mirabegron may vary depending on patient characteristics such as age, comorbidities, and severity of OAB symptoms. The cost of medications and healthcare services may vary across different countries and healthcare systems. There is a scarcity of literature in the Indian setting on pharmacoeconomic evaluation of mirabegron as compared to solifenacin in OAB. The objective of this study was to determine the cost analysis of mirabegron as compared to solifenacin in the treatment of OAB syndrome.
MATERIAL AND METHODS
This was a secondary analysis of data done during the electives posting of undergraduate students in the Department of Pharmacology. The data were obtained from the Department of Urology as a part of a postgraduate thesis comparing the effectiveness of mirabegron versus solifenacin at the Government Medical College in Central Kerala from December 2019 to June 2021 (IRB number: 112/2019/ GMCK dated October 18, 2019).[4] This pharmacoeconomic evaluation was done from the patient’s perspective for a short time horizon of 3 months. Consecutive patients were prescribed solifenacin 5 mg or mirabegron 25 mg once daily at night and OAB-V-8 score was used for comparing the effectiveness of the drugs at baseline and at 12 weeks. Of the 298 patients recruited in the study, each received either mirabegron or solifenacin. Soliten and Mirago (both from Sun Pharmaceutical Industries Ltd.) the most prescribed brands were considered for the estimation of cost of solifenacin and mirabegron, respectively, from https://www.1mg.com. The total costs of each drug were calculated for 90 days (cost of single drug × 90 × 149) during the time of effectiveness study and at the time of pharmacoeconomic analysis. The projected cost for 10 years was calculated assuming a constant compound annual growth rate (CAGR) using formula ([Cost of the drug in 2024/Cost of the drug in 2021](1/3))-1 and the projected cost for the year 2031 was calculated using the formula Cost in 2024 × (1 + CAGR).[5] Considering the average inflation rate of 5.5% in India from 2021 to 2024, the adjusted CAGR was calculated.
Percentage change in OAB-V-8 scores from baseline and at the end of 12th week was determined using the formula OAB V80-OAB V812/OAB V80 and multiplied by 100. The average effectiveness was determined by dividing the total percentage change by 149 for each drug. The average cost-effectiveness ratio was calculated using the cost of drug/ resulting effect and incremental cost-effectiveness ratio was to be calculated using the formula difference in costs (mirabegron-solifenacin)/difference in the effectiveness of mirabegron-solifenacin.
RESULTS
We studied the cost analysis of mirabegron versus solifenacin for the treatment of OAB syndrome for a period of 90 days. Data from 298 participants, 149 in each group, were obtained. The two groups were found to be comparable in terms of age, the mean age being 52.70 ± 11.68 years and 51.56 ± 12.39 in solifenacin and mirabegron groups, respectively, with a P = 0.41. The OAB-V-8 score at baseline was 28.07 ± 5.89 in the solifenacin group and 28.89 ± 6.50 in the mirabegron group with a P = 0.26.
The cost of a single unit of solifenacin (Soliten) 5 mg in 2021 was Rs 38 as shown in Table 1 and the total cost for treating 149 patients for 90 days in the year 2021 was Rs. 5,09,580. The current cost for treating with the same dose of solifenacin is Rs. 3,474 for a single patient for 90 days. The 10-year projected cost for a single tablet estimated using constant CAGR is Rs. 43.19 and considering 5.5% inflation is Rs. 65.51 for the year 2031.
| Drug | Unit cost (Rs.) | Cost for 90 days for 1 patient (Rs.) | Cost for 90 days for 149 patients (Rs.) |
|---|---|---|---|
| Solifenacin (Soliten) in 2021 | 38 | 3,420 | 5,09,580 |
| Solifenacin (Soliten) in 2024 | 38.6 | 3,474 | 5,17,626 |
| Solifenacin Projected cost in 2031 expecting constant CAGR | 43.19 | 3,887 | 5,79,178 |
| Solifenacin Projected cost in 2031 with 5.5% inflation | 65.51 | 5,896 | 8,54,905 |
| Mirabegron (Mirago) in 2021 | 23.9 | 2,151 | 3,20,499 |
| Mirabegron (Mirago) in 2024 | 25.3 | 2,277 | 3,39,273 |
| Mirabegron Projected Cost in 2031 expecting constant CAGR | 37.44 | 3,369 | 5,02,070 |
| Solifenacin Projected cost in 2031 with 5.5% inflation | 61.19 | 5,507 | 8,20,557 |
CAGR: Compound annual growth rate
The cost of mirabegron (Mirago) 25 mg single dose in 2021 was Rs. 23.9 and it increased to Rs. 25.3 in the year 2024. Thus, for treating 149 patients for 90 days, it was Rs. 3,20,499 in 2021, whereas in 2024 it wass Rs. 3,39,273. The projected cost for mirabegron in 2031 is estimated to be Rs. 5,02,070 and Rs. 8,20,557, respectively, considering constant growth as well as 5.5% CAGR for the study population for a period 90 days.
As shown in Figure 1, there is an incremental rise in the cost of both drugs over years and the increase for solifenacin was calculated at a CAGR of 0.86% per annum whereas that of mirabegron was calculated at 2.83% per annum. The adjusted CAGR, considering a 5.5% inflation rate per annum, was calculated for both the drugs and the projected pricing in 2031 using constant CAGR and adjusted CAGR is depicted in Figure 1.
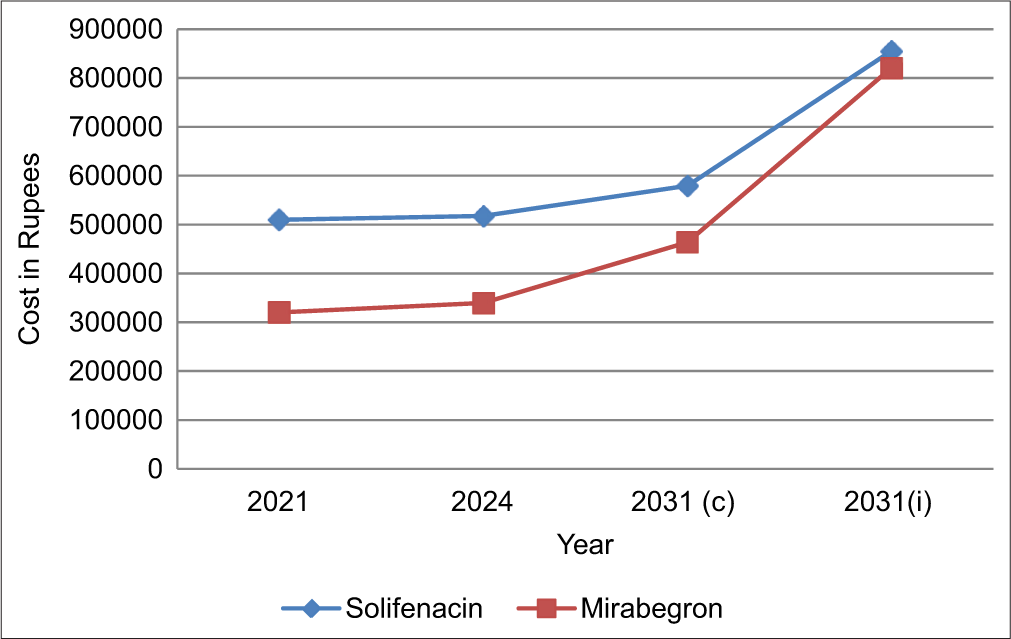
- Cost of solifenacin versus mirabegron. 2031 (c)-Pricing with constant compound annual growth rate (CAGR), 2031 (i)-Pricing with adjusted CAGR.
Table 2 summarizes the effectiveness of solifenacin and mirabegron as assessed by OAB-V-8 and there was a decline in the mean OAB-V-8 score from 28.07 to 8.05 at the end of 12 weeks while for mirabegron, it was from 28.89 to 5.74.
| Effectiveness of drug | Solifenacin | Mirabegron |
|---|---|---|
| Mean OAB V-8 at baseline (OAB-V-80) | 28.07 | 28.89 |
| Mean OAB-V-8 12 weeks (OAB-V-812) | 8.05 | 5.74 |
| Percentage change (%) | 71.32 | 80.13 |
OAB: Overactive bladder, OAB-V-8: Overactive bladder-Validated-8-question
The average cost-effectiveness ratio of solifenacin in 2021 was Rs. 7,144 per percentage decline in OAB-V-8 score. The average cost-effectiveness ratio of mirabegron was Rs. 3,999.74 per percentage change in OAB-V-8 score. This shows that mirabegron is less costly than solifenacin and it is more effective than solifenacin; hence, Incremental Cost Effectiveness ratio was not calculated as mirabegron dominates in the cost-effectiveness plane as shown in Figure 2.
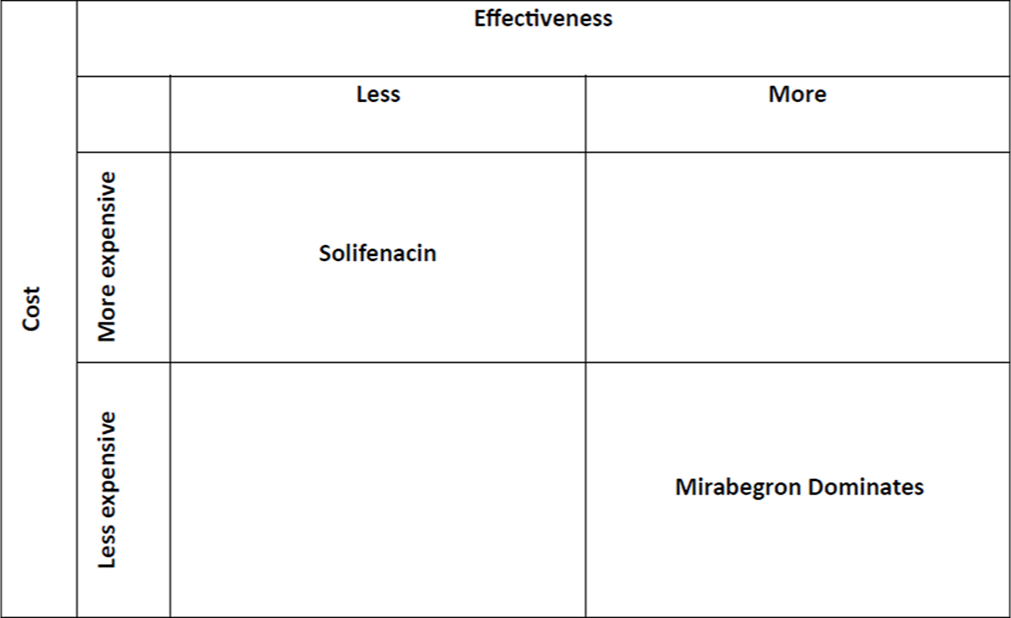
- Cost-effectiveness plane of solifenacin and mirabegron.
In neither drug group were serious adverse drug reactions (ADRs), warranting discontinuation of medication observed. While 13 patients developed ADRs such as dry mouth (n = 4) and constipation (n = 9) in the solifenacin, none were noted in those using mirabegron group. Since the ADRs were mild and non-interfering with daily routines, neither was the drug stopped, nor dose titrated to a lower dose. Since the ADRs were self-limiting, the cost of ADRs were not included. The direct non-medical costs and indirect costs like loss of wages were not included in the calculation of costs.
DISCUSSION
This pharmacoeconomic evaluation was done to determine the cost effectiveness of mirabegron versus solifenacin in OAB for a short duration of 90 days (12 weeks). This study shows that mirabegron, the newer drug as compared to solifenacin, is more effective and less costly than solifenacin. The projected costs of mirabegron and solifenacin after 10 years, based on the current costs showed that there would be a comparable increase in the costs of both solifenacin and mirabegron with only an approximate Rs. 4.32 difference based on the current CAGR and inflation rate.
Comparison of efficacy of mirabegron and solifenacin by Jamil et al. as well as Raj et al., showed that the symptoms of OAB were relieved by both solifenacin and mirabegron.[4,6] They found that mirabegron was associated with fewer treatment-related adverse events and hence advocated its use as the first-line treatment in OAB syndrome. They also opined that solifenacin may be utilized if the desired effects from mirabegron were not obtained.[6] A study was done to determine the relative efficacy and tolerability of OAB medications such as mirabegron 50 mg versus antimuscarinics such as darifenacin, tolterodine immediate release (IR), and extended release (ER), oxybutynin IR/ER, trospium, solifenacin, and fesoterodine. Bayesian mixed treatment comparisons were performed for efficacy (micturition, incontinence, UUI) and tolerability (dry mouth, constipation, blurred vision). They found that mirabegron 50 mg had similar efficacy when compared to most antimuscarinics and lower incidence of dry mouth, the most common adverse event reported with antimuscarinics and one of the main causes of discontinuation of treatment.[5] Soliman et al. evaluated the effect of mirabegron and solifenacin in children with OAB and found that both had comparable efficacy in the newly diagnosed children regarding the control of OAB symptoms and mirabegron had less side effects.[7,8] Mahapatra et al., demonstrated significant improvements in OAB symptoms while using a combination of solifenacin and mirabegron over monotherapies without increasing bothersome adverse effects associated with antimuscarinic therapy. This combination is one of the best available medical treatments for OAB irrespective of the cost considerations providing maximum efficacy and minimal side effects.[9] Behavioral therapy such as pelvic floor muscle training and delayed voiding, which is recommended as first-line therapy in OAB when combined with pharmacotherapy, optimizes the treatment of OAB .[10] Onabotulinumtoxin A was found to be the cost effective among the different interventions such as anticholinergics, β3-adrenoceptor agonists, onabotulinumtoxin A, sacral nerve stimulation, and percutaneous tibial stimulation for the treatment of OAB.[11]
Kolbin et al., opined that using mirabegron as first-line or second-line treatment for OAB was cost-effective and profitable medical technology.[12] When modeled for a 1-year horizon they found that the lowest cost was for mirabegron strategy which was 16% lower than solifenacin and comparison as second line showed 61% lower as compared to botulinum type A. Budget impact analysis as well as cost-effectiveness analysis depicted an increase in the efficiency of mirabegron compared to solifenacin strategy and reducing the burden on the budget.[12] From the UK National Health Service (NHS) perspective, mirabegron 50 mg appeared to be cost-effective when compared with oral antimuscarinic agents for the treatment of adults with OAB. They performed probabilistic sensitivity analyses and found that with willingness-to-pay threshold of mirabegron had £20,000 per quality-adjusted life years (QALY) gained and cost-effectiveness ranged from 70.2% versus oxybutynin 10 mg to 97.8% versus darifenacin 15 mg.[13] Similarly, Arlandis-Guzmán et al. found that over a 5-year time horizon, the incremental cost per patient with mirabegron 50 mg as compared to tolterodine was €195.52 and €157.42, from the NHS and societal perspectives, respectively. The cost of gaining a QALY with mirabegron versus tolterodine according to the NHS and societal perspectives was € 15,432 and €12,425, respectively. Similar comparable results were obtained with fesoterodine.[14] Other studies using a probabilistic model and Monte Carlo simulation have showed positive impact in the quality of life and cost savings to the NHS in Spain and according to societal perspectives.[15,16] Perk et al. observed that using mirabegron involved moderate additional cost to a health plan and medical cost savings can offset a substantial part translated as pharmacy costs.[17]
The main limitations of this study were that there were calculations only of the direct medical costs and other costs such as direct non-medical and indirect costs were not considered. The non-calculation of costs for the management of ADRs can be justified in the context that the ADRs reported were only a few and they were mostly self-limited. The time horizon considered was only for 90 days which is short. Since OAB requires long-term treatment, more long-term data are needed to fully assess the cost-effectiveness of mirabegron compared to solifenacin.
CONCLUSION
Mirabegron is cost-effective pharmacotherapeutic agent as compared to solifenacin in the treatment of OAB as evidenced by a lower average cost-effectiveness ratio. The individual patient characteristics and healthcare systems might affect the cost-effectiveness. Further longer studies considering more details of patients as well as cost of different treatment options in terms of provider, payer, and societal perspectives need to be undertaken in the Indian settings.
Ethical approval:
The research/study was approved by the Institutional Review Board at GMC Kottayam, number 112/2019, dated 18th October 2019.
Declaration of patient consent:
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest:
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation:
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship: Nil.
References
- Overactive Bladder. 2018. Available from: https://www.ics.org/committees/standardisation/terminologydiscussions/overactivebladder [Last accessed on 2024 Oct 16]
- [Google Scholar]
- Overactive Bladder Symptom Score-Translation and Linguistic Validation in Bengali. J Family Med Prim Care. 2022;11:79-83.
- [CrossRef] [PubMed] [Google Scholar]
- Overactive Bladder Syndrome: Evaluation and Management. Curr Urol. 2018;11:117-25.
- [CrossRef] [PubMed] [Google Scholar]
- Therapeutic Effectiveness and Adverse Drug Reactions of Mirabegron Versus Solifenacin in the Treatment of Overactive Bladder Syndrome. Perspect Clin Res. 2024;15:147-51.
- [CrossRef] [PubMed] [Google Scholar]
- Comparative Efficacy and Safety of Medical Treatments for the Management of Overactive Bladder: A Systematic Literature Review and Mixed Treatment Comparison. Eur Urol. 2014;65:755-65.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of Solifenacin and Mirabegron for the Treatment of Overactive Bladder. J Ayub Med Coll Abbottabad. 2023;35:298-300.
- [Google Scholar]
- Mirabegron Versus Solifenacin in Children with Overactive Bladder: Prospective Randomized Single-Blind Controlled Trial. Urol Int. 2021;105:1011-7.
- [CrossRef] [PubMed] [Google Scholar]
- Effectiveness and Tolerability of Mirabegron in Children with Overactive Bladder: A Retrospective Pilot Study. J Pediatr Surg. 2020;55:316-8.
- [CrossRef] [PubMed] [Google Scholar]
- Solifenacin and Mirabegron Monotherapies Versus Combination Therapy in Overactive Bladder: A Prospective Observational Study. Biomed Pharmacol J. 2022;15:491-7.
- [CrossRef] [Google Scholar]
- Effectiveness of Combined Behavioral and Drug Therapy for Overactive Bladder Symptoms in Men: A Randomized Clinical Trial. JAMA Intern Med. 2020;180:411-9.
- [CrossRef] [PubMed] [Google Scholar]
- Cost-Effectiveness of Overactive Bladder Treatments from a US Commercial and Payer Perspective. J Comp Eff Res. 2023;12:e220089.
- [CrossRef] [PubMed] [Google Scholar]
- Pharmacoeconomic Analysis of Using Mirabegron to Treat Overactive Bladder in the Setting of the Russian Federation Health Care. Urologiia. 2016;1:32-9.
- [Google Scholar]
- Cost-Effectiveness of Mirabegron Compared with Antimuscarinic Agents for the Treatment of Adults with Overactive Bladder in the United Kingdom. Value Health. 2015;18:783-90.
- [CrossRef] [PubMed] [Google Scholar]
- Cost Effectiveness of Mirabegron and Antimuscarinic Drugs in Patients with Hiperactive Bladder. Arch Esp Urol. 2018;71:809-24.
- [Google Scholar]
- Analysis of Costs and Consequences Related to the Persistence of Mirabegron and Antimuscarinic Treatments and Their Impact on Quality of Life in Patients with Overactive Bladder in Spain: Results of a Probabilistic Model. Arch Esp Urol. 2020;73:509-22.
- [Google Scholar]
- Economic Impact Associated with Patients with Overactive Bladder on Drug Treatment with Mirabegron or Antimuscarinics in Spain. Arch Esp Urol. 2023;76:98-106.
- [CrossRef] [PubMed] [Google Scholar]
- Estimated Budget Impact of Increased Use of Mirabegron, A Novel Treatment for Overactive Bladder. J Manag Care Spec Pharm. 2016;22:1072-84.
- [CrossRef] [PubMed] [Google Scholar]







