Translate this page into:
Enzyme-Linked Immunosorbent Assay versus Chemiluminescent Immunoassay: A General Overview

-
Received: ,
Accepted: ,
How to cite this article: Khan M, Shah SH, Salman M, Abdullah M, Hayat F, Akbar S. Enzyme-linked immunosorbent assay versus chemiluminescent immunoassay: A general overview. Glob J Med Pharm Biomed Update 2023;18:1.
Abstract
Enzyme-linked immunosorbent assay (ELISA) technique measures antigens, antibodies, and protein reactions in biological samples by enzymatic reactions. The chemiluminescence immunoassay (CLIA) technique determines sample concentrations based on the intensity of the light emitted by a chemical and biological reaction. This review provides an overview to understand the ELSIA and CLIA methods with their types and comparison. ELISA and CLIA methods were compared based on previous literature studies. In conclusion, CLIA is found highly sensitive, specific, and rapid, as compared to ELISA, but CLIA is an expensive method as compared to ELISA.
Keywords
Enzyme-linked immunosorbent assay
Chemiluminescence immunoassay
Antigen
Antibody
Sensitivity
INTRODUCTION
Immunoassays are bioanalytical procedures that rely on the reaction of an analyte and an antibody (Ab) to determine analyte concentration. The label activity (e.g., radiation, fluorescence, or enzyme) in either the bound or free fraction is measured. Immunoassay techniques are widely employed in many critical areas of pharmaceutical analysis, including illness diagnostics, therapeutic medication monitoring, conventional medicinal, and bioequivalence investigations in the drug development and pharmaceutical industries.[1] In these immunoassay techniques, based on antigen (Ag) and Ab responses, the enzyme-linked immunosorbent assay (ELISA) is mostly used as a diagnostic tool. ELISA is utilized for the detection of numerous types of infections and diagnostic criteria due to its high sensitivity and specificity. The manual approach, on the other hand, is labor and time-intensive, making it unsuitable for speedy detection. Chemiluminescence immunoassay (CLIA) is a powerful mixture of immunoreaction and chemiluminescent technology, which has lately gained popularity in diagnostics.[2]
Herein, this review aimed to compare the ELISA with CLIA methods based on previous literature studies. This review provides an overview to understand the ELSIA and CLIA methods with their types and comparison.
ELISA
ELISA is a widely used immunological method for determining the presence of Ags, Abs, and proteins in biological samples. Pregnancy testing, HIV infection detection, and the measurement of soluble receptors or cytokines in serum are just a few examples.[3] ELISA tests are frequently done in plates of 96-wells because a single experiment allows multiple sample measuring. The Ag or Ab is attached to the surface of plates due to their particularly absorbent capability. For the detection of each Ag or Ab, various types of kits are commercially available.[4]
Principle of ELISA
An ELISA’s core premise is to use an enzyme to determine Ag and Ab binding. The colorless substrate is transformed with the help of an enzyme into a colorful product, suggesting that Ag: Ab binding is present. Depending on how the test or which type of ELISA is constructed, an ELISA can detect the presence of Ags or Abs in a sample.[5,6]
Types of ELISA
Based on Ag or Ab binding onto the well’s surface of the ELISA plates or detection of Ag or Ab, the ELISA is divided into the direct, indirect, sandwich, and competitive ELISA methods [Figure 1].

- ELISA types and its principle. ELISA: Enzyme-linked immunosorbent assay.
Direct ELISA
In the initial step of both direct and indirect ELISA, the plates cover with Ag. In a direct ELISA, the primary recognition Ab binds directly to the interest or the analyte. After the substrate addition, the plate is rewashed for the removal of any unattached antibodies. Due to the availability of rarer procedures for indirect ELISA, it avoids cross-reactivity of the secondary Ab and is quicker than indirect ELISA. In comparison with other types of ELISA, this type has low sensitivity and is costly.[3,7]
Indirect ELISA
Except for an extra wash step and the kinds of antibodies introduced after the buffer is removed, the indirect ELISA processes are identical to the stages of the direct ELISA. Two antibodies are required in indirect ELISA: One is a primary recognition Ab (attaches to the target protein) and the second is a subsequent enzyme-linked Ab (acts in conjunction with the main Ab). After applying the primary Ab, a step of washing is accomplished, and then, the enzyme-bound with secondary Ab is introduced and incubated. The phases after that were the same as a direct ELISA, including a wash phase, substrate introduction, and color change observation.[8] In comparison with the direct ELISA method, indirect ELISA has a high sensitivity. It is also cost-effective and more versatile (various primary antibodies used). The chance of cross-reactivity among the antibodies is the main disadvantage of this type.[4]
Sandwich ELISA
This type of ELISA starts with an attached Ab inserted in the plate wells. Sandwich ELISA compromises the best sensitivity in comparison to other types of ELISA methods because the antibodies two-layer sandwiched the Ags (harvest and recognition antibodies). The main drawbacks of this form of ELISA are the time and expansiveness, as well as the utilization of matched pairs and secondary antibodies.[7]
Competitive ELISA
Ag-specific antibodies are recognized in the test serum using a competitive ELISA. This kind of ELISA uses two antibodies, one enzyme-conjugated and the second detected in the positive test sample. The two antibodies will compete for Ag binding in the wells if they are mixed. In the negative test, the color changes are noted which is the indication of the conjugation of enzyme-bound antibodies and Ags, whereas if the color does not change, it is the indication of the Abs presence, meaning that the test is positive. Because competitive ELISA has low specificity, it cannot be used with dilute samples. However, the benefits include minimum sample purification, the ability to test a wide range of Ag s in a single sample, the ability to use microscopic Ags, and low variability.[7,20]
CLIA
CLIA is a technique for determining sample concentrations based on the intensity of the light emitted by a chemical and biological reaction. The chemiluminescence (CL) systems and immunoreactions are combined in CLIA. Some chemicals have been used as CL labels, and the system generates chemiluminescence when the CL substrates are added, allowing the samples to be measured. The most frequent CL substrates include luminol, their derivatives, alkaline phosphatase (ALP), peroxidase, and acridinium ester compounds. In CLIA, the enzyme is also used for the markup of the target proteins. ALP and horseradish peroxidase are widely used for enzyme labeling.[21] CLIA has been applied in a variety of fields, including clinical diagnosis, environmental monitoring, pharmaceutical analysis, and food safety. CLIA offers the benefits of high CL sensitivity and immunoreaction specificity.[22]
Principle of CLIA
To detect the tiny biological molecules for immunoassay, enzyme-labeled antibodies and Ags are used. The approach works on the idea that an Ag binds to a specific Ab in immunology. Ag molecules such as hormones, peptides, and proteins can be detected in a fluid sample. The enzymes used in the chemiluminescent microparticle immunoassay convert a substrate to a reaction product, which generates a photon of light instead of producing a distinct color. When a material transitions from an excited to a ground state, it emits light called luminescence [Figure 2].[23]

- Chemiluminescence immunoassay and its principle.
ELISA VERSUS CLIA
In the comparison of ELISA and CLIA, the CLIA is highly sensitive. ELISA measures optical density, whereas CLIA measures relative light unit. Based on the detection range, the CLIA range is high as compared to ELISA. The CLIA is a rapid test, while the ELISA is time-consuming. The ELISA is a cost-effective test as compared to CLIA [Figure 3]. Besides these comparisons, the previous studies also reported that the CLIA is better as compared to ELISA. The previously reported studies on the comparison of ELISA and CLIA are presented in [Table 1].
| Research Title | Results | References |
|---|---|---|
| Comparison of SARS-CoV-2 serological tests with different Ag targets. | ELISA and CLIA both tests had a sensitivity of 90% and a specificity of 98% | [8] |
| A comparison of 7 commercial anti-SARS-CoV-2 Ab immunoassays. | CLIA and ELISA was 92.3% for IgG and 75.0% for IgA | [9] |
| Head-to-head comparison of ELISA and ECLIA for the detection of TTD Markers; HIV, HCV, and HBV in blood donors, in India. | Both tests were 100% sensitive and specific | [10] |
| Diagnostic accuracy of serological tests for COVID-19: Systematic review and meta-analysis. | CLIA sensitivity for IgG or IgM was 97.8% and ELISA was 84.3% | [11] |
| Comparative evaluation and measure of accuracy of ELISAs, CLIAs, and ECLIAs for the detection of HIV infection among blood donors in China. | Both ELISA and CLIA were more specific and accurate | [12] |
| Comparison of CLIA, ELISA and passive agglutination for diagnosis of Mycoplasma pneumoniae infection. | CLIA highly specific and sensitive, in comparison to ELISA | [2] |
| Comparison between ELISA and CLIA for the detection of Hepatitis C virus Ab. | Sensitivity of ELISA was 96.07%, while the sensitivity of CLIA was 96.66%. Their results also suggest that CLIA early detected the infection of HCV as compared to ELISA. | [13] |
| Performance evaluation of CLIA for Treponema pallidum specific antibodies detection in comparison with ELISA. | CLIA was more reliable, accurate, and sensitive compared with ELISA | [14] |
| Comparing assay performance of ELISA and CLIA in detecting antibodies to hepatitis B surface Ag. | Coefficient-of-variation of ELISA was 74.5% and CLIA was 113.1% | [15] |
| Comparison of three different methods for the quantification of equine insulin. | Accuracy of ELISA was 98±4%, and for CLIA was 101±11% | [16] |
| The clinical performance of a chemiluminescent immunoassay in detecting anti-cardiolipin and anti-β2 glycoprotein I antibodies. A comparison with a homemade ELISA method. | CLIA was high specific as compared with homemade ELISA | [17] |
| Comparison of chemiluminescent immunoassay and ELISA for measles IgG and IgM. | CLIA showed high sensitivity and specificity as compared with ELISA | [18] |
| Comparison for ELISA and CLIA of serum 25-hydroxy vitamin D determination. | Both CLIA and ELISA results are the same | [19] |
ELISA: Enzyme linked immunosorbent assay, ECLIA: Enhanced chemiluminescence immunoassay, TTD: Transfusion transmitted disease, CLIA: Chemiluminescence immunoassay
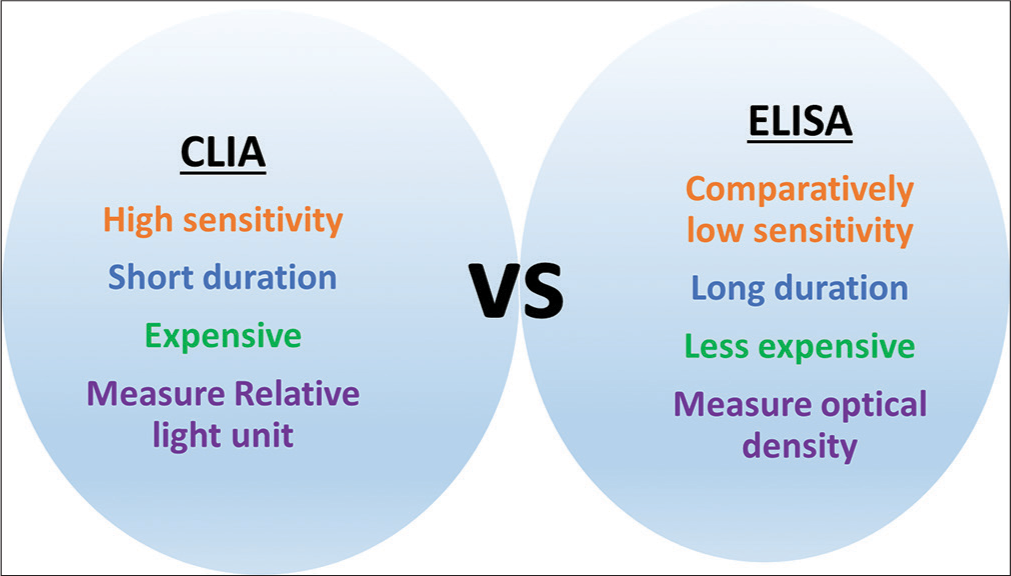
- Comparison of ELISA and CLIA. ELISA: Enzyme-linked immunosorbent assay and CLIA: Chemiluminescence immunoassay.
CONCLUSION
CLIA is a better alternative approach for detecting Ag or antibodies than conventional tests, notably ELISA. In comparison to ELISA, it can assist in detecting early infection and is ideal for laboratories with large sample volumes.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Conflicts of interest
There are no conflicts of interest.
Financial support and sponsorship
Nil.
References
- Immunoassay methods and their applications in pharmaceutical analysis: Basic methodology and recent advances. Int J Biomed Sci. 2006;2:217-35.
- [Google Scholar]
- Comparison of chemiluminescence immunoassay, enzyme-linked immunosorbent assay and passive agglutination for diagnosis of Mycoplasma pneumoniae infection. Ther Clin Risk Manag. 2018;14:1091-7.
- [CrossRef] [PubMed] [Google Scholar]
- Enzyme-linked immunosorbent assay (ELISA): The basics. Br J Hosp Med. 2016;77:C98-101.
- [CrossRef] [PubMed] [Google Scholar]
- Enzyme-Linked Immunosorbent Assay Treasure Island (FL): StatPearls Publishing; 2021.
- [Google Scholar]
- Direct competitive enzyme-linked immunosorbent assay (ELISA) Cold Spring Harb Protoc. 2017;2017:pdb.prot093740.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of SARS-CoV-2 serological tests with different antigen targets. J Clin Virol. 2021;134:104690.
- [CrossRef] [PubMed] [Google Scholar]
- A comparison of 7 commercial anti-SARS-CoV-2 antibody immunoassays. Arch Med Sci. 2020;16:1-8.
- [CrossRef] [Google Scholar]
- Head-to-head comparison of Enzyme Linked Immunosorbent Assay (ELISA) and Enhanced Chemiluminescence Immunoassay (ECLIA) for the detection of Transfusion Transmitted Disease (TTD) Markers; HIV, HCV and HBV in blood donors, in India. J Virol Methods. 2020;285:113962.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnostic accuracy of serological tests for covid-19: Systematic review and meta-analysis. BMJ. 2020;370:m2516.
- [CrossRef] [PubMed] [Google Scholar]
- Comparative evaluation and measure of accuracy of ELISAs, CLIAs, and ECLIAs for the detection of HIV infection among blood donors in China. Can J Infect Dis Med Microbiol. 2020;2020:2164685.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison between ELISA and chemiluminescence immunoassay for the detection of Hepatitis C virus antibody. Indian J Microbiol Res. 2017;4:3JA.
- [Google Scholar]
- Performance evaluation of CLIA for Treponema pallidum specific antibodies detection in comparison with ELISA. J Clin Lab Anal. 2015;25:21839.
- [CrossRef] [PubMed] [Google Scholar]
- Comparing assay performance of ELISA and chemiluminescence immunoassay in detecting antibodies to hepatitis B surface antigen. J Clin Diagn Res. 2016;10:DC22-5.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of three different methods for the quantification of equine insulin. BMC Vet Res. 2016;12:196.
- [CrossRef] [PubMed] [Google Scholar]
- The clinical performance of a chemiluminescent immunoassay in detecting anti-cardiolipin and anti-β2 glycoprotein I antibodies. A comparison with a homemade ELISA method. Clin Chem Lab Med. 2015;53:1083-9.
- [CrossRef] [Google Scholar]
- Comparison of chemiluminescent immunoassay and ELISA for measles IgG and IgM. APMIS. 2015;123:648-51.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison for ELISA and CLIA of serum 25-hydroxy vitamin D determination. Wei Sheng Yan Jiu. 2015;44:435-9.
- [Google Scholar]
- The ELISA, enzyme-linked immunosorbent assay. Clin Chem. 2010;56:319-20.
- [CrossRef] [PubMed] [Google Scholar]
- Chemiluminescent immunoassay and its applications. Chin J Anal Chem. 2012;40:3-10.
- [CrossRef] [Google Scholar]
- Flow-injection chemiluminescent immunoassay for α-fetoprotein based on epoxysilane modified glass microbeads. J Immunol Methods. 2006;312:61-7.
- [CrossRef] [PubMed] [Google Scholar]
- A recent review on chemiluminescence reaction, principle and application on pharmaceutical analysis. Inter Sch Res Not. 2013;2013:230858.
- [CrossRef] [Google Scholar]






