Translate this page into:
Stem Cell Therapy in Parkinson’s Disease: Advances in Regenerative Medicine and Clinical Applications

*Corresponding author: Devika Sanil Kumar, Department of Research, Panimalar Medical College Hospital and Research Institute, Chennai, Tamil Nadu, India. devikasds2980@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Kumar DS, Ravi R, Razzak Mahmood AA, Amirkhanyan N, Polevoy GG. Stem Cell Therapy in Parkinson’s Disease: Advances in Regenerative Medicine and Clinical Applications. Glob J Med Pharm Biomed Update. 2025;20:2. doi: 10.25259/GJMPBU_42_2024
Abstract
Parkinson’s disease (PD) is a neurodegenerative disorder characterized by the gradual loss of dopaminergic neurons in the substantia nigra, resulting in both motor and non-motor symptoms. The current treatment options, including medications and surgical procedures, primarily focus on symptom management without addressing the underlying progression of the disease. Stem cell therapy has emerged as a promising approach in regenerative medicine for PD, aiming to replace the damaged neurons, restore dopamine production, and enhance overall patient outcomes. Furthermore, the review discusses the latest developments in clinical trials, evaluating the therapeutic potential as well as the limitations of stem cell-based treatments. While preclinical studies have demonstrated encouraging results, the translation of these findings into effective clinical treatments remains a complex challenge. The diverse nature of PD, varying patient responses, and long-term safety concerns emphasize the need for ongoing research. This review provides an in-depth analysis of stem cell therapies in PD, addressing their therapeutic potential, sources, and reprogramming techniques. It critically examines key challenges such as graft purity, safety, immunological rejection, and complications like dyskinesia. By exploring advancements in neural and dental pulp stem cells, the review underlines the role of PD microenvironment in influencing outcomes and highlights cutting-edge reprogramming approaches such as episomal and polycistronic vectors and helps to gain a comprehensive understanding of current innovations, limitations, and future directions in stem cell-based treatments for PD.
Keywords
Stem cell
Regenerative medicine
Parkinson’s disease
Neural stem cells
INTRODUCTION
L-Dopa remains a widely used treatment for managing Parkinson’s disease (PD) symptoms, though its effectiveness tends to decline over time, leading to severe side effects such as dyskinesia. Prenatal cell transplantation has shown promise in significantly slowing the progression of PD.[1] However, due to ethical and legal concerns, the use of fetal tissue in transplants has decreased, necessitating the exploration of alternative methods for obtaining dopamine (DA)-producing neurons.[2] Stem cells are increasingly recognized as a valuable source of biological material for therapeutic purposes, especially in the context of neurological disorders like PD, which currently lack long-lasting and effective treatments. Cultured under precise conditions, stem cells provide a reliable source of living tissue for medical applications. The potential of stem cells as an innovative treatment strategy is evident in successful transplants for PD.[3] For instance, stem cells could supply viable tissue for cell-based therapies targeting neurological disorders such as PD, where existing treatments are inadequate. The capacity of stem cells to be precisely controlled and cultured offers nearly limitless possibilities for generating therapeutic tissue. The creation of induced pluripotent stem cells (iPSCs) through the introduction of embryogenesis-associated genes into adult somatic cells, such as skin fibroblasts, represents a breakthrough. In addition, the identification of iPSCs and the ability to differentiate various tissue-specific stem cells from highly specialized cells are significant scientific achievements. Medical research has explored various types of stem cells, including neural stem cells (NSCs), adipose-derived stem cells, bone marrow-derived mesenchymal stem cells (MSCs), and stem cells isolated from the bloodstream during sepsis. NSC lines that have been genetically modified to include oncogenes demonstrate continuous proliferation, offering valuable insights for basic research on stem cell replacement, gene therapy, and brain development.
Stem cell therapies in PD
Research has consistently demonstrated the potential of stem cell therapies, such as cell transplantation, for treating PD.[4] The primary strategies for applying stem cells in neurodegenerative diseases involve replacing lost or damaged cells or utilizing their autocrine and paracrine effects to stimulate the body’s neural progenitors to produce essential neurotrophic and developmental factors.[5] Typically, endogenous brain progenitor cells show limited differentiation capabilities. However, introducing stem cells can enhance their proliferation and maturation, restoring the necessary conditions for cell viability and activating external neural progenitor cells more effectively.[6] Transplanted stem cells emit a variety of bioactive exosomes, neurotrophic factors, and cytokines essential for healing injured nerve tissue. Peripheral nerve cells can produce essential molecules such as neurotrophin-3, glial-derived neurotrophic factor, neurotrophic factor from the brain, and glucose development factor, thanks to these secretions, which can help with nerve regeneration. These bioactive substances can also facilitate neurogenesis, reduce cell death, modulate inflammation, and support the formation of connections between damaged neurons.[7]
Role and potential of NSCs in neurogenesis
The breakthrough in neuroscience involving the identification of NSCs in adult brains, after their initial discovery in embryos, is a significant achievement. These adult NSCs are predominantly located in the hippocampus and the subventricular zone, which are key regions for neurogenesis in adults. Moreover, the presence of NSCs has been observed in certain regions of the spinal cord, which are generally not associated with the generation of new neurons. The outcomes of cultured stem cells can be influenced by the growth factors used during the culture process. For instance, the introduction of epidermal growth factor (EGF) into the subventricular zone can enhance cell proliferation and change their migration from a parallel to a radial direction, resulting in the differentiation of cells into the glial lineage rather than neurons.[8] These NSC populations have been identified in two specific regions of the brain: The sub granular zone within the dentate gyrus of the hippocampus and the subependymal zone along the ventricular walls.[9] These cells continue to divide until they lose their stem cell characteristics and differentiate into astrocytes, neurons, and other cell types. NSCs are vital for the regeneration of injured DA neurons by initiating differentiation processes. In treating PD, these NSCs, which can be obtained from both adult and fetal central nervous system (CNS) tissues, hold significant therapeutic potential.
PD’s microenvironment
Stopping the progression of the disease by treating the underlying brain damage is the main objective of PD therapy. Stem cell therapy might not outperform DA replacement therapy if the transplanted neurons are limited to only replacing the lost DA neurons. Research indicates that the microenvironment around the graft can be adversely affected. In PD patients, disease progression has been observed in grafted neurons, leading to the formation of Lewy bodies. It suggests that α-synuclein, a protein associated with PD, might spread from the host to the grafted neurons, promoting further aggregation and pathology. The Prion hypothesis explains that α-synuclein aggregates, like prions, can propagate through the nervous system. By causing soluble α-synuclein to aggregate after it adopts a β-sheet shape, α-synuclein can cause Lewy illness.[10,11] The dispersion and disintegration of these mature clumps may initiate a cycle of neural aggregation. Although grafted DA neurons show potential in animal models with neurotoxin-induced damage, their survival in a human brain remains uncertain due to the persistent adverse environment in PD. It influences the need to improve the harmful microenvironment, which could be aided by understanding PD mechanisms through iPSC PD models. Research has demonstrated that human NSC grafts can create more astrocytes, which secrete neuroprotective substances and help regulate the microenvironment.[12] Considering the different microenvironments in different patients and the limited efficacy of simply replacing DA neurons, future directions might involve developing a self-sufficient DA “unit.”[13] To help PD patients cope with their unfavorable surroundings and restore lost DA neurons, this unit may contain glial cells, growth factors, and DA neurons. Moreover, ensuring the graft’s autonomy in controlling cell numbers is crucial, as it would allow for better management of the graft’s exposure to the microenvironment and enhance the overall effectiveness of the therapy.
Sources of pluripotent stem cells
Researchers are looking into various cell sources to reprogram cells into iPSCs. The use of peripheral blood cells is particularly promising due to the minimal invasiveness and low risk associated with drawing blood. Drawing blood is a routine procedure with established safety protocols, making it an appealing choice for generating iPSCs. During the procedure, a small amount of blood must be drawn to separate particular cell types transformed into immature pluripotent stem cells (iPSCs). This method simplifies the procedure and reduces the discomfort and potential complications associated with more invasive cell collection techniques, thus enhancing the feasibility and appeal of using peripheral blood cells for research and therapeutic applications. Another notable advancement in the field is reprogramming keratinocytes, cells found in hair follicles, into iPSCs.[14] However, this method requires plucking a substantial amount of hair, which may need to be more convenient for donors. Looking toward the future, there is significant potential to improve accessibility by developing techniques to generate iPSCs from a simple mouth swab. This approach simplifies the collection process, making it easier for individuals to participate in stem cell research and therapy.
Embryonic stem cells (ESCs)
ESCs are characterized by their ability to self-renew and differentiate into any of the three primary germ layers: Ectoderm, mesoderm, and endoderm. These cells are extracted from the inner cell mass of a blastocyst during early development. Human ESCs (hESCs), derived from pre- or peri-implantation embryos, are distinct from their murine counterparts as they can differentiate into cells from all three germ layers even after being cultured for extended periods.[15] This potential makes hESCs a valuable option for therapeutic use in PD, particularly in generating reliable DA progenitors and neurons. Scientists employ various methods to steer ESCs toward specific brain cell types, such as administering morphogens like retinoic acid and sonic hedgehog, co-culturing with feeder cells, and applying genetic alterations. These techniques have proven effective in producing DA neurons that can address functional deficits in PD animal models. Advances in differentiating midbrain DA neurons from ESCs may involve using specific transcription factors like nuclear receptor related 1 protein (NURR1) and LIM homeobox transcription factor 1 alpha (LMX1a) or combining different differentiation strategies.[16] Recently, more efficient and streamlined protocols have been developed for differentiating ESCs into DA neurons, relying exclusively on chemically defined additives such as sonic hedgehog (SHH), fibroblast growth factor (FGF)8, recombinant human noggin, and dibutyryl-cAMP.[17] These methods remove the need for feeder cells and genetic modifications. The differentiation process is guided by a combination of transcription factors (such as orthodenticle homeobox 2, LMX1a, and NURR1) and signaling molecules (such as SHH, WNT, and FGF8), which together direct the formation of midbrain DA neurons. A recent study demonstrated the more efficient generation of tyrosine hydroxylase positive (TH+) neurons with the A9 phenotype in a reduced timeframe. The use of signaling molecules with precise regulation of factors, and sometimes feeder cells, can further enhance the induction of DA neurons from ESCs. Barker et al. developed a technique to produce a high yield of DA neurons from mouse ESCs in vitro. They expanded undifferentiated stem cells and selected CNS stem cells using FGF-2. Differentiation into TH-positive DA neurons was achieved by withdrawing the mitogen, although no transplantation was carried out. Overexpressing the transcription factor Nurr-1 in mouse ESCs significantly improved the yield of DA neurons. These neurons expressed DA markers, released DA, and exhibited typical electrophysiological characteristics. When transplanted into the rat striatum, these cells survived, extended processes, and alleviated PD-like symptoms [Figure 1].[18]

- Embryonic Stem Cells (Under usage license Creative Commons Attribution 4.0 International).
MSCs
MSCs are multifunctional cells usually present in the bone marrow, although they can also be found in the dermis, fatty tissue, blood from the peripheral organs, and the umbilical cord. These cells can differentiate into many lineages, such as osteocytes, chondrocytes, and adipocytes. MSCs can undergo trans differentiation into neurogenic cells like nestin-positive neurospheres when exposed to factors such as EGF and basic FGF (bFGF).[19] MSCs from peripheral blood with neurogenic potential are being explored for autologous transplantation in treating neurodegenerative diseases. Given that PD results in the death of DA neurons, MSCs may provide a promising cell replacement therapy. MSCs can be categorized into naive and induced types, both showing promise for PD treatment. Studies have investigated MSCs from various tissues in PD models, demonstrating their ability to promote functional recovery and, in some cases, develop neuronal characteristics. Neurally-induced bone MSCs (BMSCs) have shown improved survival, TH expression, and behavioral benefits in PD models, with neurally-induced BMSCs showing particularly strong effects.[20] In addition, in models of Parkinson’s illness, MSCs generated from adipose tissue and the umbilical cord have demonstrated promising outcomes.
DA neuron stem cells
Mesencephalic tissue from human embryos has been traditionally used in cell replacement therapies for PD. Although this approach has shown limited clinical efficacy, it faces significant ethical and logistical issues, including the need for tissue from multiple fetuses per patient. Recent research indicates that achieving therapeutic benefits requires a substantial quantity of dissociated DA cells. Studies using non-dissociated mesencephalic tissue without immunosuppressive treatments and with fewer DA neurons have not demonstrated significant therapeutic effects.[21] An alternative strategy involves grafting tissues that include various catecholamine-producing cells, such as chromaffin or carotid body cells, rather than directly attempting to replace lost cells. While chromaffin cells have been largely abandoned due to poor long-term outcomes, carotid body glomus cells show promise for treating preclinical models of PD.[22] Numerous methods have been devised to generate L-DOPA and DA, such as utilizing neural progenitor cells or multipotent stem cells to attain a functioning DA phenotype in the midbrain. DA phenotyping NSCs is a practical tactic. For instance, NSCs derived from non-ventral mesencephalon that has been genetically or epigenetically modified to become immortalized have shown potential.[23] Similar techniques have generated a DA phenotype in human NSCs from the embryonic forebrain at 5–11 weeks of gestation.[24] These cells, when grown in a medium supplemented with heparin, leukemia inhibitory factor, bFGF, and EGF, formed neurospheres in suspension culture. A few DA neurons were produced when plated on polyornithine-coated surfaces and cultured with interleukin (IL)-1b, either alone or with IL-11, GDNF, and IL-11. However, these neurons’ extent, properties, and grafting efficacy have not been thoroughly investigated. While iPSCs offer the potential for PD cell-based therapy, their complex generation and differentiation procedures and the risk of tumor formation from undifferentiated cells limit their clinical application. As an alternative, directly generating DA neurons from somatic cells, such as fibroblasts, presents significant promise. Recent studies have successfully reprogrammed fibroblasts from PD patients into DA neurons (induced DA [iDA] neurons) using various combinations of transcription factors. Smidt et al. used eight factors to determine that Acsl1 and Pitx3 are essential for this process, demonstrating that these neurons function in a PD mouse model.[25]
Graft purity
The composition of grafts is critical in PD transplantation. However, it remains uncertain whether grafts combining glial cells and pure DA neurons provide superior symptom relief. Evidence suggests that astrocytes play a role in brain development, indicating that glial cells may influence the behavior of precursor cells after they are implanted.[26] Consequently, mesencephalic tissues containing glial cells are commonly used in transplants.[27] Among these, A9 Substantia Nigra neurons, which project to the striatum, are considered more beneficial compared to A10 Ventral Tegmental Area neurons.[28] The subtype of DA neurons is also significant. When human embryonic or fetal ventral mesencephalic tissues were transplanted into Parkinson’s patients in the late 1980s, the outcomes were not entirely satisfactory. While some open-label trials, such as those by Madrazo and Lindvall, reported improvements in the Unified PD Rating Scale, the overall use of transplants for PD has been debated due to ethical concerns and mixed findings from National Institutes of Health-funded double-blind trials in the 1990s, which revealed adverse effects and limited clinical benefits.[29]
iPSCs generated by non-integrating viral vectors
Generating iPSCs from human and mouse somatic cells often involves using viruses to deliver essential transcription factors. However, since viral DNA can be incorporated into the host genome, potentially disrupting genetic processes and increasing the risk of cancer, there are concerns. Replication-defective adenoviruses and other non-integrating viral vectors were developed in response to these concerns. These adenoviruses can genetically alter fibroblasts to produce transient expression of reprogramming genes and the development of pluripotent cells that do not incorporate into the host DNA. The Forschungsbericht von Stadtfeld and Zhou show how iPSCs from humans and mice can successfully develop into all three germ layers, including DA neurons, without including viral DNA.[30] The removable Sendai Virus (SeV), which does not incorporate into the host genome, has also been used to create transgene-free iPSC lines. Developing a temperature-sensitive, non-integrating SeV Vector (SeV TS7) has further advanced this field by enabling the production of iPSCs without the need for feeder cells and addressing safety issues associated with xenogenic or allogeneic materials.[31] Moreover, a temperature-sensitive SeV vector has facilitated reprogramming human terminally differentiated T cells into iPSCs, reducing both transgene expression and residual SeV.[32] These advancements highlight the potential of non-integrating viruses, such as adenovirus and SeV, for generating iPSCs without exogenous gene integration, emphasizing the shift towards epigenetic reprogramming and non-viral methods.
Plasmid-based reprogramming
Plasmids were among the first non-viral vectors explored as alternatives to viral vectors for generating transgene-free iPSCs. This process resulted in iPSCs without plasmid integration, which, although less effective in reprogramming than viral vectors, still could form teratomas and contribute to adult chimeras. Recent studies have shown that transient transfection with standard plasmids can generate iPSCs without insertional mutagenesis, offering a simpler approach for further refinement.[33] Although plasmid-based iPSC methods are less efficient than other techniques, further development is needed before these methods can be considered for clinical applications in treating PD.
Episomal vector
The novel non-viral technique known as episomal iPSC reprogramming, which leverages the Epstein-Barr virus (EBV), is designed to create iPSCs devoid of transgenes and viruses within a feeder-free environment. This method uses an oriP/EBNA1-based vector system consisting of three plasmids and facilitates transfection without requiring viral packaging.[34] Initially, this technique to reprogram human foreskin fibroblasts yielded fewer iPSCs compared to viral vector approaches.[35] The vector was successfully utilized to reprogram fibroblasts and EBV-immortalized B cell lines from individuals with various diseases; however, challenges were encountered in including all reprogramming-related transgenes.[36] Subsequent improvements, such as omitting c-Myc and reducing the number of reprogramming factors, have enhanced the method’s efficiency. These developments indicate the potential for using iPSCs in therapeutic applications for PD, as they can be generated without continuous reprogramming agents or genomic integration.
Polycistronic vectors in the Cre-loxP system
A new non-viral strategy has been developed to generate iPSCs without integrating transgenes. This approach involves using polycistronic vectors to introduce multiple transcription factors and removing the reprogramming cassette through Cre recombinase-mediated site-specific excision.[37] This method has effectively produced iPSCs from human and mouse fibroblasts without relying on external reprogramming factors. The study reported that Cre recombinase messenger RNA (mRNA) can remove the reprogramming cassette.[38] Furthermore, iPSCs free from viral reprogramming factors have been generated in patients with idiopathic PD using Cre-recombinase excisable constructs.[39] These iPSCs are capable of differentiating into DA neurons. However, there are ongoing concerns about potential insertional mutations from any remaining vector sequences.
Challenges in reprogramming efficiency
Effective reprogramming of iPSCs using non-viral vectors remains problematic due to low-efficiency rates. While RNA and protein-based approaches offer innovative alternatives, they are less effective. Currently, the most effective method involves viral vectors, such as lentiviral vectors, which, although more efficient, carry the risk of insertional mutagenesis.[40] Future research should focus on optimizing reprogramming techniques to enhance iPSC generation. Factors such as delivery vectors, culture conditions, and the type of somatic cells used significantly impact the yield of viable iPSCs. Further reprogramming and differentiation methods refinement is necessary before iPSCs can be clinically applied to treat PD. Moreover, carefully considering administration routes and ensuring graft purity is critical for successful outcomes [Figure 2].
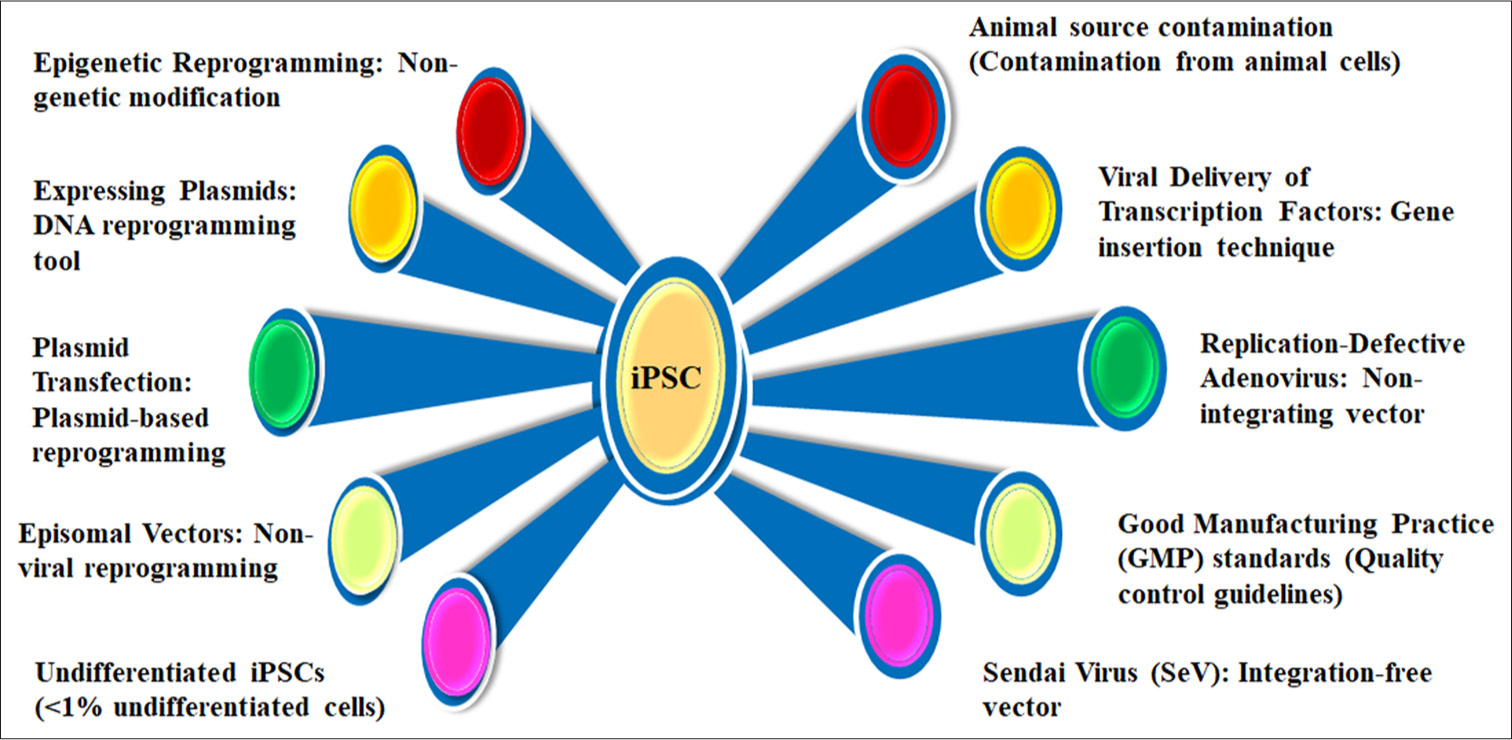
- Influential factors for induced pluripotent stem cell. iPSC: Induced pluripotent stem cells.
Figure 2 details influential factors in iPSC regeneration, emphasizing epigenetic reprogramming and non-genetic modifications for safe cellular transformation. Key methods include DNA reprogramming tools, plasmid-based techniques, and non-viral approaches, alongside viral delivery systems like replication-defective adenovirus and SeV. It highlights Good Manufacturing Practice (GMP) standards ensuring clinical-grade quality and addresses challenges such as maintaining undifferentiated iPSCs, animal source contamination, and minimizing integration risks. Together, these factors advance the safety, efficiency, and applicability of iPSC-based regenerative therapies.
Dental pulp stem cells (DPSCs) therapy for PD
In 2000, Gronthos and colleagues identified DPSCs while studying dental pulp cells. DPSCs are unique compared to bone marrow MSCs due to their ability to form in vitro colonies and fibroblast-like morphology.[41] While DPSCs express surface markers such as CD73, CD90, and CD105, they do not have markers like CD14, CD34, and CD45. DPSCs are more proficient in generating mineralized tissue than BMSCs, and they exhibit higher cell proliferation rates and frequent formation of cell clusters. In addition, DPSCs can express ESC markers such as octamer-binding transcription factor 4 (OCT4), SRY-box transcription factor 2 (SOX2), and myelocytomatosis viral oncogene (MYC), which are rarely seen in MSCs.[42] Under the right conditions, these cells can differentiate into various cell types, including neural cells, adipocytes, hepatocytes, and osteoblasts, and show potential for immune system modulation and unexpected differentiation sites within organisms.[43] DPSCs originate from the outer embryonic layer and are derived from adaptable neural crest cells.[44] They can exhibit basic nerve lineage markers such as glial fibrillary acidic protein, intermediate filament nestin, low-affinity nerve growth factor receptor p75, and more complex markers such as nuclear antigen and β-III tubulin.[45] DPSCs can also mature into neurons, assist in axon guidance, and produce and release neurotrophic factors, underscoring their potential as a promising approach for PD cell transplantation therapy.[46] Research on DPSCs has demonstrated their capacity for neuronal differentiation in vitro based on cell morphology and early neuronal markers.[47] In vivo studies support these findings, showing that DPSCs can survive and express neuronal markers following brain injection.[48] Their intrinsic ability to differentiate independently strongly indicates their potential for nerve regeneration therapies [Figure 3].
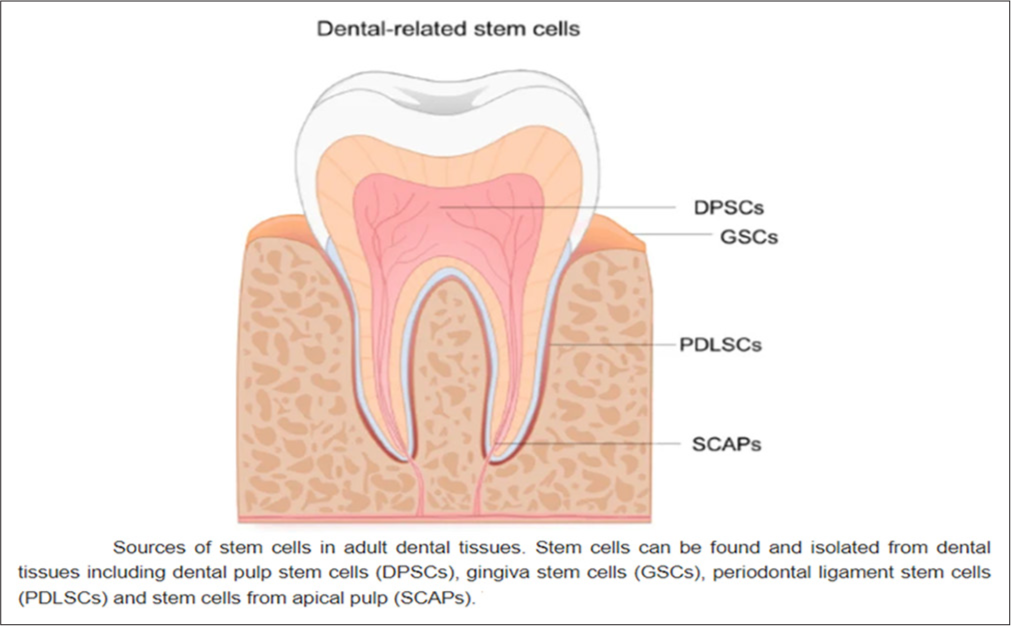
- Stem cells can be isolated from adult dental tissues such as dental pulp stem cells, gingiva stem cells, periodontal ligament stem cells, and stem cells from apical pulps, excluding developing teeth.
Neural grafts leading to dyskinesia in PD
Severe dyskinesias during “off ” periods were observed in 15% of patients following brain transplants, with a notable increase in these jerky and uncontrolled movements after surgery as the effectiveness of medication diminished.[49] Dyskinesia was first documented post-transplant approximately 1 year after the procedure, and it was observed in 56% of cases.[50] Clinical trials using surgical techniques might have involved fewer transplanted cells than earlier, more successful research.[51] Further studies have indicated that dyskinesia typically did not emerge until after the cessation of immunosuppressive treatment, suggesting that immunosuppression could significantly influence its development.[52] Another concern is the presence of diverse grafts, including serotonergic neurons, in the ventral putamen.[53] These grafts have been associated with the formation of nerve regeneration clusters and improper DA production.[54] Consequently, it is recommended to transplant a sufficient number of cells that consist of a homogeneous group of DA neurons in the basal ganglia, alongside the use of immunosuppression, to prevent the occurrence of graft-induced dyskinesia.[55] Despite this, severe dyskinesias were a significant issue for only a small number of patients undergoing clinical treatment. There was no clear correlation between the severity of dyskinesias and the extent of DA reinnervation from the graft or the improvement of symptoms. Therefore, severe dyskinesias should not be seen as an inevitable consequence of replacing DA neurons and should not hinder the progress of developing cell-based treatments for PD. A deeper understanding of the underlying mechanisms of DA neuron transplantation is crucial to prevent “off ”-phase dyskinesias following brain transplantation. Recent studies indicate that dyskinesias may be more influenced by the host brain’s response to the graft and the integration of new neurons rather than the extent of DA reinnervation itself.[56] Factors such as the precise placement of the graft, the type of DA neurons used, and the interaction between grafted neurons and the host environment are critical to the clinical outcome. Advanced imaging techniques and electrophysiological studies are essential in tracking the functional integration of grafted neurons, allowing for real-time monitoring of neuronal activity and synaptic connections. These methods provide insights into the dynamics of neuronal circuitry post-transplantation. Moreover, molecular and genetic profiling of both donor and host tissues can identify biomarkers predictive of favorable outcomes, enabling the optimization of cell preparation protocols and grafting techniques. Innovative strategies, including stem cell-derived DA neurons and gene-editing technologies, are being explored to enhance the efficacy and safety of cell-based therapies for PD.[57]
Immunological rejection
Despite the brain being an immune-privileged site, grafts can still provoke an adverse immune response from the host. The interaction between the innate immune system and the implanted cells plays a critical role in determining the survival of the transplant.[58] Insufficient immunosuppression during transplantation, as observed in various clinical trials, has been linked to unfavorable outcomes. The surgical procedure can compromise the blood-brain barrier, reducing the brain’s immune-privileged status and potentially allowing immune cells to infiltrate. Therefore, immunosuppressive therapies are crucial to prevent graft rejection and ensure the viability and integration of the transplanted cells. One effective strategy to minimize immunological rejection involves generating hiPSC lines from a donor with matched human leukocyte antigen genes in both copies. While this approach shows promise in reducing immune-mediated rejection in allogeneic transplantation, challenges may arise, particularly in individuals with diverse ethnic backgrounds. Studies have shown that immunosuppressive medications such as azathioprine, cyclosporine, and prednisolone are associated with improved transplant outcomes.[59] However, patient conditions can deteriorate when immunosuppressive treatment is discontinued.[60] Post-mortem examinations have revealed evidence of immune responses, with transplanted tissues surrounded by active microglia.[61] These findings underscore the importance of maintaining immunosuppressive therapy alongside grafting, as immune responses can significantly impact the success of transplantation. Further research is needed to determine the most effective immunosuppressant and treatment regimen.
Concern of tumourigenesis
The risk of tumor formation has been a longstanding concern among researchers, particularly about iPSCs. The oncogenes Myc and Klf4, commonly employed in iPSC reprogramming, are at the core of this problem. Several genes, such as Nanog, Sox2, and Oct4, integral to iPSC generation, have also been linked to tumor growth.[62] A significant connection exists between iPSC production and the tumor suppressor gene p53, which has garnered considerable attention.[63] Another mechanism that can lead to tumorigenesis in iPSCs is the disruption of genomic integrity, which may occur due to somatic mutations or viral integration. Several methods have been devised to produce insertion-free iPSCs, guaranteeing the maintenance of genomic integrity during reprogramming to avoid such problems. These methods include SeV vectors, episomal vectors, and synthetic modified mRNA.[64] Maintaining genome integrity remains a critical challenge, particularly in cases where retroviral insertion has not visibly damaged the genome. Various reprogramming methods, including non-integrating approaches, have revealed mutations in iPSCs, often in cancer-associated genes. These mutations tend to be non-synonymous, nonsense, or splice variants. Consequently, strict screening protocols are necessary when using iPSC-derived neurons in therapeutic research, even with extensive measures to mitigate tumor risks. This screening includes in vivo testing, where neuron grafts are implanted in monkey models to identify any novel mutations that might arise during reprogramming, alongside comprehensive genetic analysis.
Safety and purity stem cells
To enable the effective use of DA neurons or iPSC-derived NSCs in PD treatment, it is critical to ensure that the remaining percentage of undifferentiated iPSCs in the sample is kept under 1% [Figure 3]. This precaution is necessary to prevent teratoma formation after transplantation.[65] Techniques such as fluorescence-activated cell sorting and other non-invasive magnetic selection methods have been developed to categorize iPSC-derived cells. Moreover, to eliminate the risk of contamination from animal sources, it is essential to maintain cell cultures in a feeder-free environment.[66] Traditionally, many feeder cells used for the cultivation of human iPSCs (hiPSCs) and hESCs are derived from mice and are typically grown in media containing fetal bovine serum. This practice can lead to contamination of iPSC-derived cells with allogeneic cells. However, recent findings indicate that feeder cells are not required to generate hiPSCs or hESCs when using StemFit™ medium.[67] This development marks significant progress in producing therapeutic-grade cells that meet GMP standards [Figure 4].
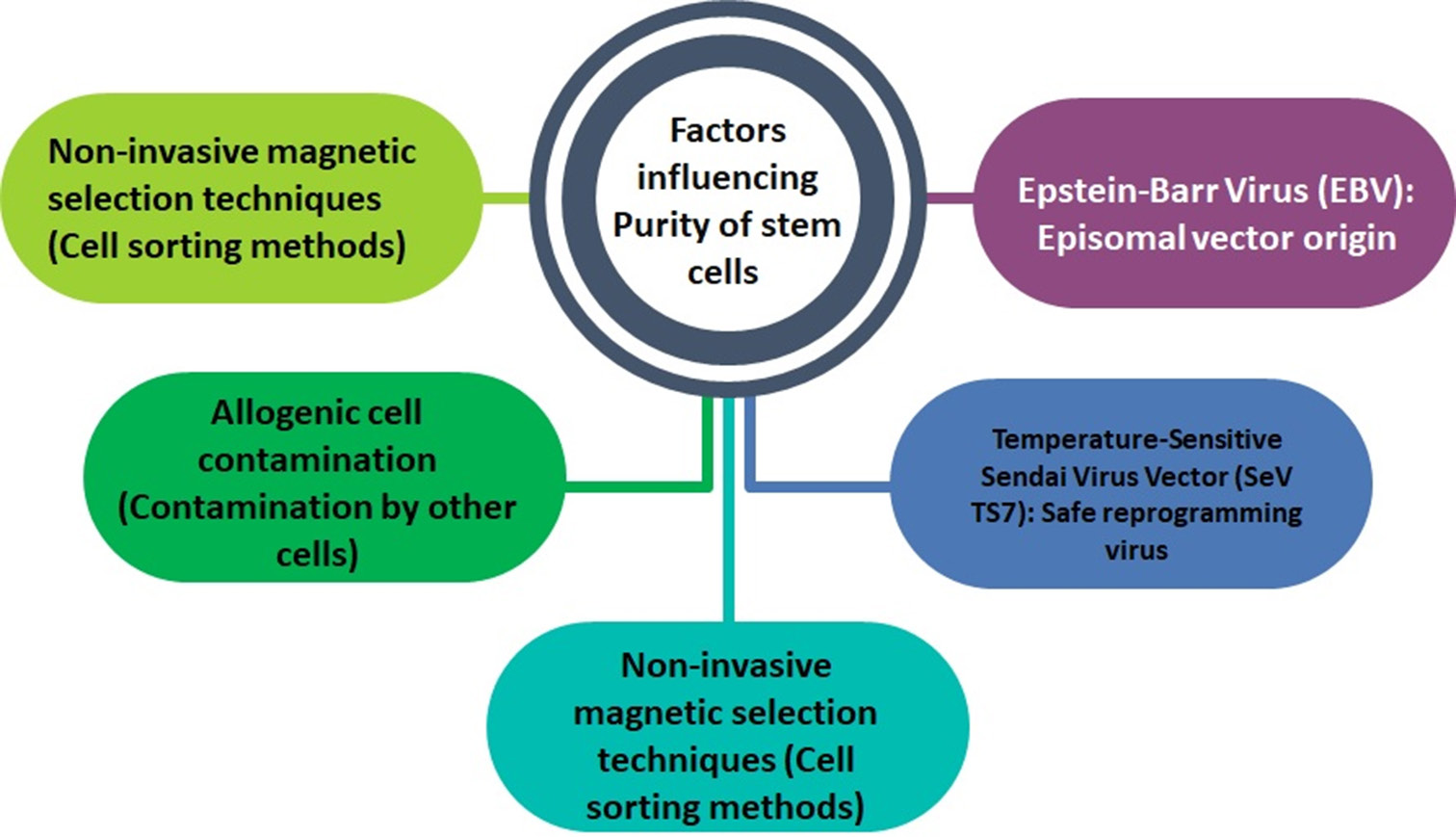
- Factors influencing the purity of stem cells.
Genetic abnormalities
The persistence of epigenetic memory in iPSCs and iDAs neurons remains uncertain, particularly due to their origins and the possible retention of reprogramming signatures post-differentiation. To express reprogramming genes, it is advisable to use non-integrating vectors rather than lentivirus or retrovirus-based methods.[68] These vectors can also be used with small-molecule drugs to generate iPSCs with therapeutic potential. Some iPSCs derived from PD patients may exhibit genetic anomalies, including point mutations, chromosomal structural changes, gene duplications, and deletions in genes such as SNCA, Parkin, LRRK2, and GBA.[69] Due to the disruption of key biological functions caused by these genetic abnormalities, iPSC-derived cells may not be ideal for direct transplantation. Several methods have been devised to correct mutations in pluripotent stem cells sourced from individuals with PD. Results imply that a zinc-finger nuclease-mediated strategy may be used to correct the SNCA mutation (A53T) in iPSCs.[70] Patient-derived iPSCs are still able to develop into DA neurons despite this modification. The correctness of the patient-derived corrected iPSC lines has been verified by PCR genotyping and sequencing analysis.[71] Furthermore, a recent study has shown that LRRK2 G2019S mutant correction in iPSCs effectively restored normal properties to neurons previously impacted by the mutation.[72]
CONCLUSION
Advancements in stem cell research provide new therapeutic possibilities for individuals who resist conventional treatment methods. It is achieved by creating patient-specific pluripotent stem cells that can develop into many kinds of cells both ex vivo and in vivo. It is critical to continue fundamental and applied research to have a thorough knowledge of the causes of PD. Using these findings, we can develop innovative and sophisticated treatments that target both the physical and non-physical symptoms of PD.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship: Nil.
References
- Long-term Clinical Outcome of Fetal Cell Transplantation for Parkinson Disease: Two Case Reports. JAMA Neurol. 2014;71:83-7.
- [CrossRef] [PubMed] [Google Scholar]
- Intravenous Transplantation of Adipose-Derived Mesenchymal Stem Cells Promoted the Production of Dopaminergic Neurons and Improved Spatial Memory in A Rat Model of Parkinson's Disease. Cell J. 2023;25:317-26.
- [Google Scholar]
- Mechanism of Mesenchymal Stem Cells as a Multitarget Disease-Modifying Therapy for Parkinson's Disease. Curr Neuropharmacol. 2023;21:988-1000.
- [CrossRef] [PubMed] [Google Scholar]
- Stem Cell Transplantation Therapy in Parkinson's Disease. Springerplus. 2015;4:597.
- [CrossRef] [PubMed] [Google Scholar]
- MiR-210-5p Promotes the Differentiation of Human-induced Pluripotent Stem Cells into Dopaminergic Neural Precursors by Targeting SMAD4 and SUFU and Treating Parkinsonian Rats. Exp Gerontol. 2023;179:112243.
- [CrossRef] [PubMed] [Google Scholar]
- Preclinical and Dose-ranging Assessment of hESC-derived Dopaminergic Progenitors for a Clinical Trial on Parkinson's Disease. Cell Stem Cell. 2024;31:278-9.
- [CrossRef] [PubMed] [Google Scholar]
- Human Umbilical Cord-derived Mesenchymal Stem Cells-harvested Mitochondrial Transplantation Improved Motor Function in TBI Models by Rescuing Neuronal Cells from Apoptosis and Alleviating Astrogliosis and Microglia Activation. Int Immunopharmacol. 2023;118:110106.
- [CrossRef] [PubMed] [Google Scholar]
- Single-Cell Sequencing Of Human Midbrain Reveals Glial Activation and a Parkinson-specific Neuronal State. Brain. 2022;145:964-78.
- [CrossRef] [PubMed] [Google Scholar]
- Inhibiting Microglia-Derived NLRP3 Alleviates Subependymal Edema and Cognitive Dysfunction in Posthemorrhagic Hydrocephalus after Intracerebral Hemorrhage via AMPK/Beclin-1 Pathway. Oxid Med Cell Longev. 2022;2022:4177317.
- [CrossRef] [PubMed] [Google Scholar]
- The Synaptic Pathology of Alpha-synuclein Aggregation in Dementia with Lewy Bodies, Parkinson's Disease and Parkinson's Disease Dementia. Acta Neuropathol. 2010;120:131-43.
- [CrossRef] [PubMed] [Google Scholar]
- The Involvement of Dityrosine Crosslinking in α-synuclein Assembly and Deposition in Lewy Bodies in Parkinson's Disease. Sci Rep. 2016;6:39171.
- [CrossRef] [PubMed] [Google Scholar]
- Astrocytes Migrate from Human Neural Stem Cell Grafts and Functionally Integrate into the Injured Rat Spinal Cord. Exp Neurol. 2019;314:46-57.
- [CrossRef] [PubMed] [Google Scholar]
- Transplantation of Human Neural Stem Cells in a Parkinsonian Model Exerts Neuroprotection via Regulation of the Host Microenvironment. Int J Mol Sci. 2015;16:26473-92.
- [CrossRef] [PubMed] [Google Scholar]
- Induced Pluripotent Stem Cells in Parkinson's Disease: Scientific and Clinical Challenges. J Neurol Neurosurg Psychiatry. 2016;87:697-702.
- [CrossRef] [PubMed] [Google Scholar]
- Human Adult Marrow Cells Support the Prolonged Expansion of Human Embryonic Stem Cells in Culture. Stem Cells. 2003;21:131-42.
- [CrossRef] [PubMed] [Google Scholar]
- Engineering a Dopaminergic Phenotype in Stem/Precursor Cells: Role of Nurr1, Glia-derived signals, and Wnts. Ann N Y Acad Sci. 2005;1049:51-66.
- [CrossRef] [PubMed] [Google Scholar]
- Induction and Specification of Midbrain Dopaminergic Cells: Focus on SHH, FGF8, and TGF-beta. Cell Tissue Res. 2004;318:23-33.
- [CrossRef] [PubMed] [Google Scholar]
- Stem Cells and Acellular Preparations in Bone Regeneration/Fracture Healing: Current Therapies and Future Directions. Cells. 2024;13:1045.
- [CrossRef] [PubMed] [Google Scholar]
- Expression of EGF Receptor and FGF Receptor Isoforms During Neuroepithelial Stem Cell Differentiation. J Neurobiol. 1999;38:207-24.
- [CrossRef] [Google Scholar]
- Human Gingival Mesenchymal Stem Cells Improve Movement Disorders and Tyrosine Hydroxylase Neuronal Damage in Parkinson's Disease Rats. Cytotherapy. 2022;24:1105-20.
- [CrossRef] [PubMed] [Google Scholar]
- Rat Fetal Ventral Mesencephalon Grown As Solid Tissue Cultures: Influence of Culture Time and BDNF Treatment on Dopamine Neuron Survival and Function. Brain Res. 1998;813:313-22.
- [CrossRef] [PubMed] [Google Scholar]
- Adrenal Chromaffin Cells as Transplants in Animal Models of Parkinson's Disease. J Electron Microsc Tech. 1989;12:308-15.
- [CrossRef] [PubMed] [Google Scholar]
- Brain Transplantation of Immortalized Human Neural Stem Cells Promotes Functional Recovery in Mouse Intracerebral Hemorrhage Stroke Models. Stem Cells. 2007;25:1204-12.
- [CrossRef] [PubMed] [Google Scholar]
- Specification of a Dopaminergic Phenotype from Adult Human Mesenchymal Stem Cells. Stem Cells. 2007;25:2797-808.
- [CrossRef] [PubMed] [Google Scholar]
- Homeobox Gene Pitx3 and Its Role in the Development of Dopamine Neurons of the Substantia Nigra. Cell Tissue Res. 2004;318:35-43.
- [CrossRef] [PubMed] [Google Scholar]
- The Emerging Roles of Transplanted Radial Glial Cells in Regenerating the Central Nervous System. Neural Regen Res. 2015;10:1548-51.
- [CrossRef] [PubMed] [Google Scholar]
- Unilateral Transplantation of Human Fetal Mesencephalic Tissue into the Caudate Nucleus of Patients with Parkinson's Disease. N Engl J Med. 1992;327:1541-8.
- [CrossRef] [PubMed] [Google Scholar]
- Induction of A9 Dopaminergic Neurons from Neural Stem Cells Improves Motor Function in an Animal Model of Parkinson's Disease. Brain. 2008;131:630-41.
- [CrossRef] [PubMed] [Google Scholar]
- Designing Stem-cell-based Dopamine Cell Replacement Trials for Parkinson's Disease. Nat Med. 2019;25:1045-53.
- [CrossRef] [PubMed] [Google Scholar]
- Non-integrating Methods to Produce Induced Pluripotent Stem Cells for Regenerative Medicine: An Overview In: Biomechanics and Functional Tissue Engineering. London: IntechOpen; 2009.
- [Google Scholar]
- Efficient Generation of Transgene-free Human Induced Pluripotent Stem Cells (iPSCs) by Temperature-sensitive Sendai virus Vectors. Proc Natl Acad Sci U S A. 2011;108:14234-9.
- [CrossRef] [PubMed] [Google Scholar]
- Sendai virus Vector-mediated Transgene Expression in the Cochlea in vivo. Audiol Neurootol. 2007;12:119-26.
- [CrossRef] [PubMed] [Google Scholar]
- Efficient Transfection of Embryonic and Adult Stem Cells. Stem Cells. 2004;22:531-43.
- [CrossRef] [PubMed] [Google Scholar]
- Establishment of an oriP/EBNA1-based Episomal Vector Transcribing Human Genomic β-globin in Cultured Murine Fibroblasts. Gene Ther. 2002;9:1447-54.
- [CrossRef] [PubMed] [Google Scholar]
- Direct Reprogramming to Human Induced Neuronal Progenitors from Fibroblasts of Familial and Sporadic Parkinson's Disease Patients. Int J Stem Cells. 2019;12:474-83.
- [CrossRef] [PubMed] [Google Scholar]
- Reprogramming of EBV-Immortalized B-lymphocyte Cell Lines into Induced Pluripotent Stem Cells. Blood. 2011;118:1801-5.
- [CrossRef] [PubMed] [Google Scholar]
- Site-Specific Recombinase Strategy to Create Induced Pluripotent Stem Cells Efficiently with Plasmid DNA. Stem Cells. 2011;29:1696-704.
- [CrossRef] [PubMed] [Google Scholar]
- RNA-generated and Gene-edited Induced Pluripotent Stem Cells for Disease Modeling and Therapy: RNA-Generated and Gene-Edited iPSCs. J Cell Physiol. 2017;232:1262-9.
- [CrossRef] [PubMed] [Google Scholar]
- iPSC-Derived Microglia as a Model to Study Inflammation in Idiopathic Parkinson's Disease. Front Cell Dev Biol. 2021;9:740758.
- [CrossRef] [PubMed] [Google Scholar]
- Insertional Mutagenesis by Retroviral Vectors: Current Concepts and Analysis Methods. Curr Gene Ther. 2013;13:211-27.
- [CrossRef] [PubMed] [Google Scholar]
- Lentiviral Vector-based Insertional Mutagenesis Identifies Genes Involved in the Resistance to Targeted Anticancer Therapies. Mol Ther. 2014;22:2056-68.
- [CrossRef] [PubMed] [Google Scholar]
- Neural Basis of Dental Pulp Stem Cells and its Potential Application in Parkinson's Disease. CNS Neurol Disord Drug Targets. 2022;21:62-76.
- [CrossRef] [PubMed] [Google Scholar]
- Bone-marrow-derived Mesenchymal Stem Cell Therapy for Neurodegenerative Diseases. Expert Opin Biol Ther. 2009;9:1487-97.
- [CrossRef] [PubMed] [Google Scholar]
- Stem Cell Therapy in Neurodegenerative Diseases: From Principles to Practice. Neural Regen Res. 2012;7:1822-31.
- [Google Scholar]
- Hepatic Stellate Cells Express the Low Affinity Nerve Growth Factor Receptor p75 and Undergo Apoptosis in Response to Nerve Growth Factor Stimulation. Am J Pathol. 2000;156:1235-43.
- [CrossRef] [PubMed] [Google Scholar]
- Neural Stem Cell Transplantation Promotes Functional Recovery from Traumatic Brain Injury via Brain Derived Neurotrophic Factor-Mediated Neuroplasticity. Mol Neurobiol. 2018;55:2696-711.
- [CrossRef] [PubMed] [Google Scholar]
- Neural Crest-Derived Stem Cells (NCSCs) Obtained from Dental-Related Stem Cells (DRSCs): A Literature Review on Current Knowledge and Directions toward Translational Applications. Int J Mol Sci. 2022;23:2714.
- [CrossRef] [PubMed] [Google Scholar]
- Intranasal Administration of Human MSC for Ischemic Brain Injury in the Mouse: In vitro and In vivo Neuroregenerative Functions. PLoS One. 2014;9:e112339.
- [CrossRef] [PubMed] [Google Scholar]
- Dyskinesias after Neural Transplantation in Parkinson's Disease: What do We Know and What is Next? BMC Med. 2010;8:80.
- [CrossRef] [PubMed] [Google Scholar]
- Neural Grafting in Parkinson's Disease Unraveling the Mechanisms Underlying Graft-induced Dyskinesia. Prog Brain Res. 2010;184:295-309.
- [CrossRef] [PubMed] [Google Scholar]
- Surgical Therapy for Parkinson's Disease. Eur J Neurol. 2002;9(Suppl 3):31-9.
- [CrossRef] [PubMed] [Google Scholar]
- Immunosuppression by Mesenchymal Stem Cells: Mechanisms and Clinical Applications. Stem Cell Res Ther. 2010;1:2.
- [CrossRef] [PubMed] [Google Scholar]
- Cell Replacement Therapy for Parkinson's Disease. Biochim Biophys Acta. 2009;1792:688-702.
- [CrossRef] [PubMed] [Google Scholar]
- Predictive Markers Guide Differentiation to Improve Graft Outcome in Clinical Translation of hESC-Based Therapy for Parkinson's Disease. Cell Stem Cell. 2017;20:135-48.
- [CrossRef] [PubMed] [Google Scholar]
- L-DOPA-and Graft-induced Dyskinesia Following Transplantation. Prog Brain Res. 2012;200:143-68.
- [CrossRef] [PubMed] [Google Scholar]
- Integrating Pathways of Parkinson's Disease in a Molecular Interaction Map. Mol Neurobiol. 2014;49:88-102.
- [CrossRef] [PubMed] [Google Scholar]
- Recent Advances in Developing Stem-cell-derived Dopaminergic Neuronal Transplant Therapies for Parkinson's Disease. Mov Disord. 2021;36:1772-80.
- [CrossRef] [PubMed] [Google Scholar]
- Is the Immunological Response a Bottleneck for Cell Therapy in Neurodegenerative Diseases? Front Cell Neurosci. 2020;14:250.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of Three Immunosuppressive Regimens in Cadaver Renal Transplantation: Long-term Cyclosporine, Short-term Cyclosporine Followed by Azathioprine and Prednisolone, and Azathioprine and Prednisolone without Cyclosporine. N Engl J Med. 1988;318:1499-507.
- [CrossRef] [PubMed] [Google Scholar]
- Discontinuation of Immunosuppression in Proliferative Lupus Nephritis: Is It Possible? Nephrol Dial Transplant. 2006;21:1465-9.
- [CrossRef] [PubMed] [Google Scholar]
- Microglial Activation in Parkinson's Disease Using [(18)F]-FEPPA. J Neuroinflammation. 2017;14:8.
- [CrossRef] [PubMed] [Google Scholar]
- Characterization of SOX2, OCT4 and NANOG in Ovarian Cancer Tumor-Initiating Cells. Cancers (Basel). 2021;13:262.
- [CrossRef] [PubMed] [Google Scholar]
- Linking the p53 Tumour Suppressor Pathway to Somatic Cell Reprogramming. Nature. 2009;460:1140-4.
- [CrossRef] [PubMed] [Google Scholar]
- Ultrasound Targeted CNS Gene Delivery for Parkinson's Disease Treatment. J Control Release. 2017;261:246-62.
- [CrossRef] [PubMed] [Google Scholar]
- Transplantation of Human Embryonic Stem Cell-derived Cells to a Rat Model of Parkinson's Disease: Effect of In vitro Differentiation on Graft Survival and Teratoma Formation. Stem Cells. 2006;24:1433-40.
- [CrossRef] [PubMed] [Google Scholar]
- Biomaterials for the Feeder-free Culture of Human Embryonic Stem Cells and Induced Pluripotent Stem Cells. Chem Rev. 2011;111:3021-35.
- [CrossRef] [PubMed] [Google Scholar]
- Feeder-dependent and Feeder-independent iPS Cell Derivation from Human and Mouse Adipose Stem Cells. Nat Protoc. 2011;6:346-58.
- [CrossRef] [PubMed] [Google Scholar]
- Silencing of Gene Expression: Implications for the Design of Retrovirus Vectors. Rev Med Virol. 2001;11:205-17.
- [CrossRef] [PubMed] [Google Scholar]
- Comprehensive Analysis of the LRRK2 Gene in Sixty Families with Parkinson's Disease. Eur J Hum Genet. 2006;14:322-31.
- [CrossRef] [PubMed] [Google Scholar]
- Correction: Generation of SNCA Cell Models Using Zinc Finger Nuclease (ZFN) Technology for Efficient High-Throughput Drug Screening. PLoS One. 2021;16:e0256366.
- [CrossRef] [PubMed] [Google Scholar]
- Generation of Gene-corrected iPSC Line from Parkinson's Disease Patient iPSC Line with Alpha-SNCA A53T Mutation. Stem Cell Res. 2018;30:145-9.
- [CrossRef] [PubMed] [Google Scholar]
- Patient-specific Neuronal Networks Carrying the LRRK2 G2019S Mutation in Parkinson's Disease Reveal Early Functional Alterations that Predate Neurodegeneration. NPJ Parkinsons Dis. 2021;7:55.
- [CrossRef] [PubMed] [Google Scholar]







