Translate this page into:
Finerenone, a Novel and Safer Approach toward Management of Diabetic Kidney Disease with Heart Failure

*Corresponding author: Sarmad Iqbal, Department of Pharmacy Practice, Faculty of Pharmacy and Pharmaceuticals Science, University of Karachi, Karachi, Sindh, Pakistan. s.iqbal814@outlook.com
-
Received: ,
Accepted: ,
How to cite this article: Memon AA, Iqbal S. Finerenone, a novel and safer approach toward management of diabetic kidney disease with heart failure: Glob J Med Pharm Biomed Update 2022;17:12.
Abstract
Diabetes is the major cause of chronic and end-stage renal disease worldwide. Despite recent breakthroughs in diabetic kidney disease (DKD) therapy, there is still a significant need for more choices to enhance renal and cardiovascular outcomes. Mineralocorticoid overactivity adds to inflammation and fibrosis, which leads to the advancement of DKD. The mineralocorticoid receptor antagonists (MRAs) spironolactone and eplerenone slow the course of DKD as well as the risk of hospitalizations and death in patients with heart failure (HF) with reduced ejection fraction but their potential of causing hyperkalemia, particularly in individuals with renal dysfunction, restricts their usage. Finerenone, a new non-steroidal MRA, has showed potential cardiac and renoprotective advantages in DKD as well as has a better affinity for the mineralocorticoid receptor (MR) than eplerenone and higher selectivity for the MR than spironolactone. Studies have shown that the selective non-steroidal MRA finerenone reduces the risk of cardiovascular events and chronic kidney disease (CKD) progression in individuals with CKD and type 2 diabetes mellitus. Finerenone selectivity and higher binding affinity to the MR may lower the risk of hyperkalemia and renal dysfunction, overcoming the reluctance to initiate MRAs in patients with HF and DKD.
Keywords
Finerenone
Diabetic kidney disease
Heart failure
Diabetes
Management
INTRODUCTION
Diabetes has tremendously spread worldwide continuously affecting millions of people. The prevalence of diabetes among people aged 20–79 around the world was predicted to be 10.5% (536.6 million) in 2021 and 12.2% (783.2 million) in 2045. The cost of treating diabetes-related illnesses worldwide was estimated to be 966 billion USD in 2021 and is expected to rise to 1054 billion USD by 2045.[1] The comorbidities associated with diabetes also need to be addressed as complications may affect both small and large blood vessels, leading to organ damage like kidney disease which is a major microvascular complication.[2] Chronic kidney disease (CKD) often leads to many serious complications as end-stage renal disease and cardiovascular complication.[3] Heart failure (HF) is prevalent in diabetes as evident from various epidemiological and clinical studies from the past 20 years. The health-care outcomes in diabetic patients are poor as compared to non-diabetic patients with cardiovascular disorders,[4] indicating that diabetic patients are more prone to develop cardiovascular diseases.[5] In a multinational study involving more than 750 thousand cardiovascular and renal disease-free patients with type 2 diabetes mellitus (T2DM), association of cardiovascular and renal diseases with high mortality risks was observed. In light of all the associated risks and complications, new and improved strategies are required to be developed for the prevention of such complications.[6]
In recent years, novel therapeutic categories have been developed involving several drug classes such as sodium glucose cotransporter 2 (SGLT2) inhibitors,[7] glucagon-like peptide-1 (GLP-1) agonists,[8] dipeptidyl-peptidase-4 (DPP4) inhibitors,[9] and mineralocorticoid receptor antagonists (MRAs)[10,11] to improve cardiorenal outcomes in diabetic patients. SGLT2 inhibitors are found to be associated with decreased risks of cardiovascular events.[7] Similarly, GLP-1 agonists have also been proven to reduce cardiovascular events and renal disease progression.[12] DPP4 inhibitors have found to have beneficial effects in reducing the risk of albuminuria progression[9] but more evidence is needed regarding patients with the left ventricular systolic function and HF.[13] MRAs are also the point of focus among these novel therapies to be used as an adjunct to reduce the risk of diabetic kidney disease (DKD) and cardiovascular disease in T2DM.[14]
After the discovery of aldosterone in 1953, various synthetic steroids were produced to block the sodium uptake and excretion of potassium, leading to spironolactone discovery.[15] Spironolactone was discovered as first MRA. Being non-selective, it was shown to exert harmful effects on glucose homeostasis and hyperkalemia.[16,17] Spironolactone is also found to produce reproductive side effects,[18] due to its anti-androgenic effects.[19] Eplerenone, discovered in 2002, was the first selective mineralocorticoid receptor (MR) blocker due to its lesser side effects than spironolactone.[20] This development did not resolve the risk of developing hyperkalemia.[21,22] Clinical use of steroidal MRA due to the associated risks is limited and the need of development of non-steroidal MRA began to rise.[23]
Finerenone is a recent, a novel selective non-steroidal MRA,[24] approved in 2021 by FDA.[25] It has shown efficacy for the management of cardiorenal diseases having stronger binding potential for MR compared to eplerenone and spironolactone.[24] It is well-tolerated and shown to have more potent cardiorenal effects compared to eplerenone.[26] Incidence of hyperkalemia and other adverse events is much lower in finerenone compared to steroidal MRA.[27] This article is aimed to provide a brief review of all the published literature on PubMed and Google Scholar related to finerenone with review of its clinical efficacy to provide the medical community with a glance of all the recent advancements related to finerenone.
DISCOVERY OF FINERENONE
Dihydropyridines (DHPs) were known to have L-type calcium channel antagonizing effects. By ultra-high-throughput screening, 1,4-DHPs were found to have significant MR antagonistic activity in vitro. The major candidate was found to be DHP-1 which showed selectivity (>20-fold) over glucocorticoid receptor, androgen, and progesterone receptors. Further studies were performed due to of extremely low metabolic stability in human liver and significant L-type calcium channel interaction. A significant step forward in the development of finerenone (BAY 94-8862) was made by substituting a 4-cyano2-methoxyphenyl moiety in the naphthyridine series (conformationally frozen bioisosteres of 1,4-DHPs) for the chromanone head group which resulted in dihydronaphthyridine series [Figure 1]. More active enantiomer was found by chiral HPLC then further exploration including introduction of methyl group at C8 and the replacement of cyano group by primary amide at C3 as a final step led to the development of dihydronaphthyridine (BAY 94-8862), a potent MRA (IC50 18 nm) with excellent selectivity versus GR, AR, PR (>500-fold), and virtually no L-type Ca2+ channel activity (IC50 >10 mm). It showed a good pharmacokinetic profile (low blood clearance, long half-life of 0.014 L h1 kg1, and 8.5 h, respectively, by IV administration) as well as high oral bioavailability of 94% in rats. More natriuretic effects were observed in finerenone compared to eplerenone due to its 10-fold higher potency and more efficacy.[28]
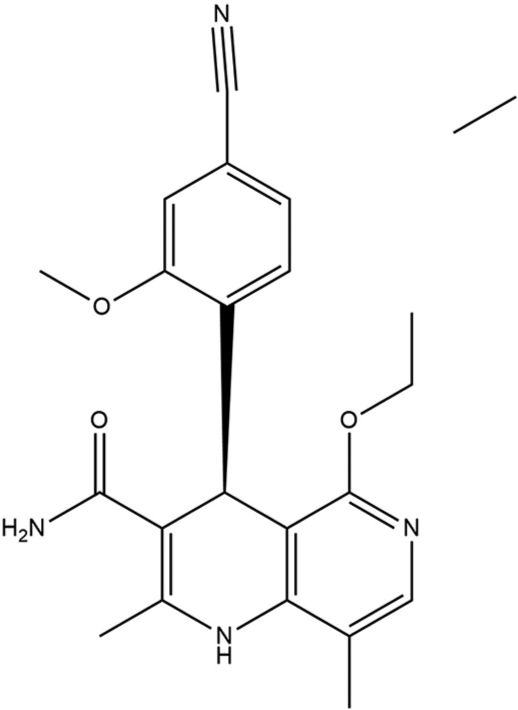
- Structure of finerenone.
PATHOPHYSIOLOGY OF DKD AND HF BY MR ACTIVATION
MR is expressed in multiple cells including cardiac myocytes, vascular endothelial cells, and vascular smooth muscle cells of kidney. Aldosterone is a steroid hormone produced in the adrenal cortex, maintains sodium and potassium balance, as well as provides blood pressure control through its MR activity. It exerts its physiological mechanism of maintaining electrolyte balance through MR binding in the connecting tubules and collecting duct in kidneys.[29] Increased oxidative stress, endothelial dysfunction, and activation of sympathetic nervous system are some of the damaging effects associated with aldosterone. It promotes myocardial fibrosis and tubular necrosis when overly expressed in heart and kidneys.[30] The activation of local MR in heart and kidney leads to cardiovascular and renal injury through aldosterone dependent and independent mechanisms [Table 1].[31]
| Mineralocorticoid receptor overactivation effects | Kidney | Heart | ||
|---|---|---|---|---|
| Impairment in mitochondrial function[32] | Leads to increase in oxidative stress[32] | |||
| Stimulation of ROS (reactive oxygen species) production in cardiac tissues by aldosterone-mediated NADPH oxidase activity[33] | Leads to cardiac fibrosis and dysfunction due to increased ROS production and changes in DNA transcription[33] | |||
| Contributes in stimulation of the inflammatory reactions involving macrophages and Th-lymphocytes[34] | The release of inflammatory substances, results in increased cardiac fibrosis further leads to cardiac dysfunction[35,36] | |||
| MR activation by aldosterone stimulates mcp-1 and the pro-inflammatory factor NF kb (nuclear factor kappa b) which further causes stimulation of growth factors for fibroblast cell proliferation in kidneys[29] | Increased oxidative stress in kidneys[29] | |||
| Activation of platelet-derived growth factor receptor and epidermal growth factor receptor[40] | Fibroblast proliferation in kidneys[40] | |||
| Epithelial mesenchymal transition is activated by aldosterone through MR-mediated pathway[42] | Interstitial fibrosis[42] | |||
Role of MR activation in HF
Although aldosterone is primarily produced in adrenal glomerulosa cells, it has extra renal effects due to the expression of MR in cardiac and vascular tissues. Glucocorticoids and aldosterone both can activate MR in cardiac tissues, but the activation is mainly achieved by glucocorticoids due to their relatively higher levels in circulation. By impairment in the mitochondrial function, MR activation can lead to increase in oxidative stress.[32] Stimulation of reactive oxygen species (ROS) production in cardiac tissues by aldosterone-mediated NADPH oxidase activity results in high myocardial oxidative stress. Altered redox-sensitive signaling pathways lead to cardiac fibrosis and dysfunction due to increased ROS production and changes in DNA transcription.[33] MR activation contributes in stimulation of the inflammatory reactions involving macrophages and Th-lymphocytes.[34] The release of inflammatory substances, growth factors, and inflammatory cytokines results in increased cardiac fibrosis which, further, leads to cardiac dysfunction.[35,36]
Role of MR activation in kidney disease
There is implication of aldosterone/MR signaling in the development of renal injury which leads to tubular necrosis.[37,38] MR activation by aldosterone stimulates MCP-1 and the pro-inflammatory factor NF Kb (nuclear factor kappa B) which, further, causes stimulation of growth factors for fibroblast cell proliferation in kidneys. Stimulation of profibrotic cytokines synthesis by aldosterone causes increased oxidative stress in kidneys.[29] Microangiopathy and angio sclerosis can take place in renal blood vessels in different ways due to MR activation.[39] Activation of platelet-derived growth factor receptor and epidermal growth factor receptor by aldosterone/MR binding results in fibroblast proliferation in kidneys.[40] Fibronectin production from renal fibroblasts is induced by aldosterone through MR-dependent JNK/cJun phosphorylation.[41] Epithelial mesenchymal transition is activated by aldosterone through MR-mediated pathway which is a key process in interstitial fibrosis.[42]
The table demonstrates the effects of MR overactivation and how it leads to heart and kidney dysfunction
FINERENONE AND OTHER MRAS
Receptor selectivity and affinity
Spironolactone is a highly potent MRA but due to its steroid receptor cross-reactivity, it is associated with serious side effects such as gynecomastia and other sexual adverse effects.[43] The first selective MRA, known as eplerenone, does not bind to progesterone and androgen receptors and, therefore, not associated with the same side effects as in spironolactone.[44] Despite these advantages, it is found to have low affinity and potency than spironolactone,[45] that is, 40-fold more potent than eplerenone.[46] Due to the limitations and potential for causing hyperkalemia, these agents have limited use in clinical practice.[28,47] Finerenone has a higher selectivity than spironolactone toward MR and higher binding affinity than eplerenone[26] and lower affinity to other receptors such as glucocorticoid, androgen, and progesterone receptors as observed in eplerenone.[48]
Drug distribution
Potential to cause hyperkalemia is exaggerated by the distribution pattern of both eplerenone and spironolactone, which are found in higher concentration in kidneys.[49] Finerenone is found to be equally distributed in cardiac and renal tissues compared to spironolactone, eplerenone, and Mespirenone in rat model. This study reflected the end-organ protective effects of finerenone due to its balanced distribution in cardiac and renal tissues while also exerting electrolyte homeostasis effects.[50]
Cardiac and renal effects
In a DOCA salt rat model of CKD, finerenone has shown reduced cardiac hypertrophy, proteinuria compared to eplerenone. Finerenone was also observed to be more effective in reducing cardiac and renal injury as well as it showed stronger inhibition of pro-fibrotic and pro-inflammatory markers than eplerenone in equinatriuretic doses.[50] According to an animal model set by Grune et al. of isoproterenol-induced cardiac fibrosis, finerenone showed antifibrotic and anti-inflammatory activity in cardiac tissues while eplerenone showed no significant effect on cardiac fibrosis. The antifibrotic activity of finerenone thought to be due to inhibition of profibrotic TNX gene expression through differential cofactor MR-binding mechanism.[51]
Molecular mode of action
Finerenone has a unique binding mode with MR which is the determinant of its selectivity, potency, and cofactor recruitment.[52] There are some major differences in the molecular mode of action of finerenone to that of other MRAs. According to the molecular modeling studies, finerenone binds MR as a bulky passive antagonist and this difference is responsible for different clinical response of finerenone as compared to other MRAs. Finerenone acts as an inverse agonist which inhibits steroid coactivator-1 recruitment opposing to spironolactone and eplerenone which act as partial agonist. Strict antagonistic activity at S810L mutant is shown by finerenone while spironolactone activates S810L mutant MR which is responsible for early-onset hypertension.[53]
Insulin resistance in obesity
In a high-fat diet-induced obesity mice model conducted by Morzolla et al., finerenone showed increased interscapular brown adipose tissue (iBAT) recruitment in rat model of remarkably high-fat diet-induced obesity which improved insulin resistance. This was confirmed by certain gene expressions which were not found in case of spironolactone showed that spironolactone had no effect on iBAT recruitment.[54]
CLINICAL PHARMACOLOGY
Finerenone mechanism of action
Finerenone is a selective MRA which is non-steroidal in structure and has higher selectivity and affinity for MR than for other receptors such as androgen, progesterone, estrogen, and glucocorticoid receptors. Overactivation of MR, which promotes fibrotic activity in heart and kidneys, leading to cardiorenal complications, is blocked by the action of finerenone in both the epithelial cells (kidney cells) and nonepithelial cells (heart and vascular tissues).[55,56] Finerenone prevents profibrotic gene transcription by preventing MR binding to coactivators.[57,58]
Pharmacokinetics, metabolism, and interactions
Finerenone has been found to be metabolized by oxidative biotransformation and lesser amounts excreted unchanged (1%) in humans. Cytochrome P450 3A4 was found to be the predominant enzyme in in vitro studies. In human plasma, the major metabolites were found to be naphthyridine compounds and they had no on-target pharmacological activity.[59] Renal function alters the clearance and other pharmacokinetic parameters of finerenone. A trial conducted on individuals of different renal function (normal, mild, moderate, and severe) showed rapid absorption of finerenone (tmax <1 h in all four groups) and no consistent effect on mean Cmax (maximum plasma concentration) in all four groups. Mean exposure area under the curve (AUC) was found to be higher in severe and moderately renally impaired patients. Elimination half-life was fast in normal renal function and mild impaired renal function compared to moderate and severe renally impaired individuals.[60] This study demonstrates that finerenone exposure is unaffected by mild renal impairment. With moderate-to-high interindividual variability and no effect on Cmax, moderate and severe renal impairment increased exposure to unbound finerenone by 57% and 47%, respectively. These findings support finerenone as a treatment in patients with chronic HF and DKD.
In a study conducted by Heinig et al., the absolute bioavailability of finerenone is found to be 43.5%, which is attributed to first-pass metabolism in the gut wall and liver with IV administration. The quantitative contribution of both isoenzymes to the metabolic clearance of finerenone that was anticipated based on in vitro research was validated by the in vivo experiments examining the functions of particular CYP3A4 and CYP2C8 inhibitors. Due to this pathway’s predominate participation, CYP3A4 inhibitors may influence finerenone exposure. Based on their CYP3A4 inhibition ratio, the examined inhibitors showed great accuracy in predicting the size of the AUC (0-∞) rise in vivo.[61] The inhibition of CYP3A4 by finerenone suggests the contraindication of concomitant administration of strong CYP3A4 inhibitors with finerenone such as rifampin, carbamazepine, phenobarbital, and phenytoin.
FINERENONE IN NEPHROPATHY AND HF
Finerenone (Kerendia®), a selective non-steroidal MRA, is developed by Bayer Healthcare Pharmaceuticals. It is approved in the USA to decrease the risk of cardiac and renal events associated with type 2 diabetes including end-stage renal disease, cardiovascular death, sustained estimated GER decline, and hospitalization for HF in CKD patients.[61,62] Finerenone has shown to decrease myofibroblast accumulation and collagen deposition in a study conducted on mice, in which kidney fibrosis was induced through ureteral obstruction. The antifibrotic activity of finerenone was accompanied by decreased kidney plasminogen activator inhibitor-1 and naked cuticle 2 expression. The antifibrotic activity was dose dependent with no effect on systemic blood pressure.[63]
HF
The MRA tolerability study (ARTS), a Phase 2A clinical trial, was designed to evaluate the tolerability and safety of finerenone in patients of HF with reduced ejection fraction (HFrEF) and mild-to-moderate CKD. The study concluded that finerenone 5–10 mg/day in patients with HFrEF and mild or moderate CKD was at least as effective as spironolactone 25 or 50 mg/day in decreasing biomarkers of hemodynamic stress and was associated with lower incidence of worsening of the kidney function and hyperkalemia.[64] A randomized, double-blind Phase 2b clinical trial called ARTS-HF was conducted to evaluate oral doses of finerenone given for 90 days in patients with worsening HF and REF and CKD and/or DM. One thousand and sixty-six patients (with type 2 DM and/or CKD presented with worsening HF and REF) were randomized in this study. For 90 days, once daily dosing of finerenone and eplerenone was administered to the patients. The results showed that finerenone was well tolerated and reduced N-terminal pro-brain natriuretic peptide levels by 30% or more in a similar proportion of patients as eplerenone. Compared to eplerenone, the composite endpoint of mortality from any cause, cardiovascular hospitalization, or emergency presentation for worsening HF occurred less frequently.[26]
CKD
The MR ARTS-Diabetic Nephropathy, a randomized, double-blind, and placebo-controlled Phase 2b study, was conducted to investigate the safety and efficacy of different doses of finerenone given for 90 days to diabetic patients with high or remarkably high albuminuria. Eight hundred and twenty-three patients were randomized to receive an angiotensin-converting enzyme inhibitor or an angiotensin receptor blocker. Out of which 36.7% of patients treated had extremely high albuminuria (UACR ≥300 mg/g) and 40.0% had an estimated glomerular filtration rate (eGFR) of ≤60 mL/min/1.73 m2 at baseline. Dose-dependent reduction of urine albumin-creatinine ratio was shown by finerenone at different doses once daily against placebo for 90 days. The study showed improvement in the urinary albumin-creatinine ratio by the addition of finerenone compared with placebo among the patients with diabetic nephropathy.[65]
An upgraded Phase 3 study, FIDELIO-DKD (finerenone in reducing kidney failure and disease progression in DKD) included 5734 patients with CKD and type 2 diabetes to receive finerenone or placebo. The study showed that finerenone, in patients with CKD and type 2 diabetes, resulted in lower risks of CKD progression (kidney failure, a sustained decrease of at least 40% in the eGFR from baseline, or death from renal causes) and cardiovascular events (death from cardiovascular causes, non-fatal myocardial infarction, non-fatal stroke, or hospitalization for HF) than placebo. Both the finerenone and placebo groups experienced a same number of adverse events during the course of therapy; patients in the finerenone group experienced major adverse events at a rate of 31.9% and 34.3%, respectively. Serious adverse events and adverse events associated with acute renal damage were distributed equally. In general, adverse events associated with hyperkalemia were twice as common with finerenone than with placebo, and hyperkalemia-related regimen termination was more common with finerenone.[66]
Finerenone in reducing cardiovascular mortality and morbidity in DKD, a large scale, randomized, double-blind, placebo-controlled Phase 3 clinical trial conducted by Filippato et al. who investigated finerenone effectiveness in preventing cardiovascular events in patients with type 2 diabetes and albuminuria from chronic renal disease. Seven thousand three hundred and fifty-two patients were included having T2DM and CKD without symptomatic HF with REF, which were randomized to finerenone or placebo. Finerenone was found to have a considerably lower risk of all HF events than placebo, including an 18% decreased risk of cardiovascular mortality. In all, 31.4% of patients receiving finerenone experienced a major adverse event, compared to 33.2% of patients receiving a placebo. The frequency of acute renal damage was comparable in all groups. Finerenone had a greater rate of hyperkalemia than the placebo (10.8% vs. 5.3%); however, none of these side effects were fatal. Hypokalemia incidence was lower with finerenone than with placebo. The results demonstrated that finerenone reduces the risk of HF outcomes and reduces the new-onset HF in T2DM and CKD patients regardless of HF history.[67,68]
CONCLUSION
Steroidal MRAs have long been used to treat refractory hypertension, and a variety of nephropathies, including diabetic nephropathy. They block receptor activation by inhibiting the action of aldosterone at the MR which reduces inflammation, inhibits remodeling, and improves proteinuria. Because these drugs are steroidal in nature, they interact with other receptors and cause undesired effects such as sexual side effects and hyperkalemia which a major reason their use is restricted. Finerenone is a new advancement in pharmacotherapy for diabetic patients with CKD and HF as it has lesser side effects and a good safety profile compared to steroidal MRAs. Additional to the typical MR inhibitory effect on fluid and electrolyte balance, Finerenone has a potent anti-inflammatory and anti-fibrotic effect. In individuals with cardiorenal disorders, these effects have been shown to be beneficial. Finerenone has shown lesser incidence of developing hyperkalemia compared to steroidal MRAs which is undoubtedly a great progress in diabetes management. Moreover, it is shown to be equipotent and have more targeted activity compared to other MRAs due to its comparatively safer therapeutics.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- IDF diabetes atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183:109119.
- [CrossRef] [PubMed] [Google Scholar]
- Mechanisms of diabetic complications. Physiol Rev. 2013;93:137-88.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical manifestations of kidney disease among US adults with diabetes, 1988-2014. JAMA. 2016;316:602-10.
- [CrossRef] [PubMed] [Google Scholar]
- Diabetes mellitus and heart failure. Am J Med. 2017;130:S40-50.
- [CrossRef] [PubMed] [Google Scholar]
- Cardiovascular protection with anti-hyperglycemic agents. Am J Cardiovasc Drugs. 2019;19:249-57.
- [CrossRef] [PubMed] [Google Scholar]
- Heart failure and chronic kidney disease manifestation and mortality risk associations in Type 2 diabetes: A large multinational cohort study. Diabetes Obes Metab. 2020;22:1607-18.
- [CrossRef] [PubMed] [Google Scholar]
- Cardiorenal outcomes with sodium/glucose cotransporter-2 inhibitors in patients with Type 2 diabetes and low kidney risk: Real world evidence. Cardiovasc Diabetol. 2021;20:169.
- [CrossRef] [PubMed] [Google Scholar]
- Diabetic kidney disease-how to protect the kidney? Dtsch Med Wochenschr. 2019;144:710-4.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of dipeptidyl peptidase-4 inhibitors on renal outcomes in patients with Type 2 diabetes: A systematic review and meta-analysis. Endocrinol Metab (Seoul). 2019;34:80-92.
- [CrossRef] [PubMed] [Google Scholar]
- Mineralocorticoid receptor antagonists and kidney diseases: Pathophysiological basis. Kidney Int. 2019;96:302-19.
- [CrossRef] [PubMed] [Google Scholar]
- The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341:709-17.
- [CrossRef] [PubMed] [Google Scholar]
- GLP-1 receptor agonists for prevention of cardiorenal outcomes in Type 2 diabetes: An updated meta-analysis including the REWIND and PIONEER 6 trials. Diabetes Obes Metab. 2019;21:2576-80.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of vildagliptin on ventricular function in patients with Type 2 diabetes mellitus and heart failure: A randomized placebo-controlled trial. JACC Heart Fail. 2018;6:8-17.
- [CrossRef] [PubMed] [Google Scholar]
- Mineralocorticoid antagonism and diabetic kidney disease. Curr Diab Rep. 2019;19:4.
- [CrossRef] [PubMed] [Google Scholar]
- The story of spironolactones from 1957 to now: From sodium balance to inflammation. G Ital Nefrol. 2016;33(Suppl 66):33.S66.12.
- [Google Scholar]
- Tolerability of spironolactone as adjunctive treatment for heart failure in patients over 75 years of age. Age Ageing. 2005;34:395-8.
- [CrossRef] [PubMed] [Google Scholar]
- A comparison of the effects of selective and non-selective mineralocorticoid antagonism on glucose homeostasis of heart failure patients with glucose intolerance or Type II diabetes: A randomized controlled double-blind trial. Am Heart J. 2018;204:190-5.
- [CrossRef] [PubMed] [Google Scholar]
- Spironolactone may be a cause of hormonally associated vestibulodynia and female sexual arousal disorder. J Sex Med. 2019;16:1481-3.
- [CrossRef] [PubMed] [Google Scholar]
- Oral spironolactone in post-teenage female patients with acne vulgaris: Practical considerations for the clinician based on current data and clinical experience. J Clin Aesthet Dermatol. 2012;5:37-50.
- [Google Scholar]
- Eplerenone: A selective aldosterone receptor antagonist for hypertension and heart failure. Heart Dis. 2003;5:354-63.
- [CrossRef] [PubMed] [Google Scholar]
- Incidence, determinants, and prognostic significance of hyperkalemia and worsening renal function in patients with heart failure receiving the mineralocorticoid receptor antagonist eplerenone or placebo in addition to optimal medical therapy: Results from the Eplerenone in Mild Patients Hospitalization and Survival Study in Heart Failure (EMPHASIS-HF) Circ Heart Fail. 2014;7:51-8.
- [CrossRef] [PubMed] [Google Scholar]
- Nonsteroidal antagonists of the mineralocorticoid receptor. Curr Opin Nephrol Hypertens. 2015;24:417-24.
- [CrossRef] [PubMed] [Google Scholar]
- Finerenone and cardiovascular outcomes in patients with chronic kidney disease and Type 2 diabetes. Circulation. 2021;143:540-52.
- [CrossRef] [PubMed] [Google Scholar]
- FDA US. Novel Drug Approvals for 2021. 2022. Silver Spring: Food and Drug Administration. Available from: https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/novel-drug-approvals-2021 [Last accessed on 2022 Jun 02]
- [Google Scholar]
- A randomized controlled study of finerenone vs. eplerenone in patients with worsening chronic heart failure and diabetes mellitus and/or chronic kidney disease. Eur Heart J. 2016;37:2105-14.
- [CrossRef] [PubMed] [Google Scholar]
- Comparative efficacy and safety of mineralocorticoid receptor antagonists in heart failure: A network meta-analysis of randomized controlled trials. Heart Fail Rev. 2019;24:637-46.
- [CrossRef] [PubMed] [Google Scholar]
- Discovery of BAY 94-8862: A nonsteroidal antagonist of the mineralocorticoid receptor for the treatment of cardiorenal diseases. ChemMedChem. 2012;7:1385-403.
- [CrossRef] [PubMed] [Google Scholar]
- Mineralocorticoid receptor activation and mineralocorticoid receptor antagonist treatment in cardiac and renal diseases. Hypertension. 2015;65:257-63.
- [CrossRef] [PubMed] [Google Scholar]
- New mineralocorticoid receptor antagonists: Update on their use in chronic kidney disease and heart failure. J Nephrol. 2020;33:37-48.
- [CrossRef] [PubMed] [Google Scholar]
- Pathophysiological roles of aldosterone and mineralocorticoid receptor in the kidney. J Pharmacol Sci. 2011;115:1-7.
- [CrossRef] [PubMed] [Google Scholar]
- Role of mineralocorticoid receptor activation in cardiac diastolic dysfunction. Biochim Biophys Acta Mol Basis Dis. 2017;1863:2012-8.
- [CrossRef] [PubMed] [Google Scholar]
- Mineralocorticoid receptor antagonism treats obesity-associated cardiac diastolic dysfunction. Hypertension. 2015;65:1082-8.
- [CrossRef] [PubMed] [Google Scholar]
- The pathophysiology of hypertension in patients with obesity. Nat Rev Endocrinol. 2014;10:364-76.
- [CrossRef] [PubMed] [Google Scholar]
- Foxp3+ CD4+ T cells improve healing after myocardial infarction by modulating monocyte/macrophage differentiation. Circ Res. 2014;115:55-67.
- [CrossRef] [PubMed] [Google Scholar]
- Uric acid promotes left ventricular diastolic dysfunction in mice fed a Western diet. Hypertension. 2015;65:531-9.
- [CrossRef] [PubMed] [Google Scholar]
- Involvement of aldosterone and mineralocorticoid receptors in rat mesangial cell proliferation and deformability. Hypertension. 2005;45:710-6.
- [CrossRef] [PubMed] [Google Scholar]
- Aldosterone and glomerular podocyte injury. Clin Exp Nephrol. 2008;12:233-42.
- [CrossRef] [PubMed] [Google Scholar]
- Aldosterone: A mediator of myocardial necrosis and renal arteriopathy. Endocrinology. 2000;141:3871-8.
- [CrossRef] [PubMed] [Google Scholar]
- Aldosterone induces kidney fibroblast proliferation via activation of growth factor receptors and PI3K/MAPK signalling. Nephron Exp Nephrol. 2012;120:e115-22.
- [CrossRef] [PubMed] [Google Scholar]
- Aldosterone stimulates fibronectin synthesis in renal fibroblasts through mineralocorticoid receptor-dependent and independent mechanisms. Gene. 2013;531:23-30.
- [CrossRef] [PubMed] [Google Scholar]
- Aldosterone induces epithelialmesenchymal transition via ROS of mitochondrial origin. Am J Physiol Renal Physiol. 2007;293:F723-31.
- [CrossRef] [PubMed] [Google Scholar]
- Spironolactone, eplerenone and the new aldosterone blockers in endocrine and primary hypertension. J Hypertens. 2013;31:3-15.
- [CrossRef] [PubMed] [Google Scholar]
- Eplerenone: A selective aldosterone receptor antagonist for patients with heart failure. Ann Pharmacother. 2005;39:68-76.
- [CrossRef] [PubMed] [Google Scholar]
- Eplerenone, a selective aldosterone blocker, in mild-to-moderate hypertension. Am J Hypertens. 2002;15:709-16.
- [CrossRef] [Google Scholar]
- The evolution of aldosterone antagonists. Mol Cell Endocrinol. 2004;217:27-31.
- [CrossRef] [PubMed] [Google Scholar]
- The use of a novel non-steroidal mineralocorticoid receptor antagonist finerenone for the treatment of chronic heart failure: A systematic review and meta-analysis. Medicine (Baltimore). 2018;97:e0254.
- [CrossRef] [PubMed] [Google Scholar]
- Non-steroidal mineralocorticoid receptor antagonism for the treatment of cardiovascular and renal disease. Eur J Heart Fail. 2017;19:811.
- [CrossRef] [PubMed] [Google Scholar]
- Molecular pharmacology of the mineralocorticoid receptor: Prospects for novel therapeutics. Mol Cell Endocrinol. 2012;350:310-7.
- [CrossRef] [PubMed] [Google Scholar]
- Finerenone, a novel selective nonsteroidal mineralocorticoid receptor antagonist protects from rat cardiorenal injury. J Cardiovasc Pharmacol. 2014;64:69-78.
- [CrossRef] [PubMed] [Google Scholar]
- Selective mineralocorticoid receptor cofactor modulation as molecular basis for Finerenone's antifibrotic activity. Hypertension (Dallas Tex: 1979). 2018;71:599-608.
- [CrossRef] [PubMed] [Google Scholar]
- Steroidal and non-steroidal mineralocorticoid receptor antagonists in cardiorenal medicine. Eur Heart J. 2021;42:152-61.
- [CrossRef] [PubMed] [Google Scholar]
- Finerenone impedes aldosterone-dependent nuclear import of the mineralocorticoid receptor and prevents genomic recruitment of steroid receptor coactivator-1. J Biol Chem. 2015;290:21876-89.
- [CrossRef] [PubMed] [Google Scholar]
- Class-specific responses of brown adipose tissue to steroidal and nonsteroidal mineralocorticoid receptor antagonists. J Endocrinol Invest. 2022;45:215-20.
- [CrossRef] [PubMed] [Google Scholar]
- The expanding class of mineralocorticoid receptor modulators: New ligands for kidney, cardiac, vascular, systemic and behavioral selective actions. Acta Endocrinol (Buchar). 2020;16:487-96.
- [CrossRef] [PubMed] [Google Scholar]
- US National Library of Medicine. 2021. Available from: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=fc726765-5d5a-4d6e-b037-b847bda9fb7c [Last accessed on 2022 Jul 09]
- [Google Scholar]
- Aldosterone and mineralocorticoid receptor signaling as determinants of cardiovascular and renal injury: From hans selye to the present. Am J Nephrol. 2021;52:209-16.
- [CrossRef] [PubMed] [Google Scholar]
- Finerenone reduces new-onset atrial fibrillation in patients with chronic kidney disease and Type 2 diabetes. J Am Coll Cardiol. 2021;78:142-52.
- [CrossRef] [PubMed] [Google Scholar]
- Biotransformation of finerenone, a novel nonsteroidal mineralocorticoid receptor antagonist, in dogs, rats, and humans, in vivo and in vitro. Drug Metab Dispos. 2018;46:1546-55.
- [CrossRef] [PubMed] [Google Scholar]
- Pharmacokinetics of the novel nonsteroidal mineralocorticoid receptor antagonist finerenone (BAY 94-8862) in individuals with renal impairment. Clin Pharmacol Drug Dev. 2016;5:488-501.
- [CrossRef] [PubMed] [Google Scholar]
- New drugs update: Finerenone, difelikefalin, and avacopan. Sr Care Pharm. 2022;37:130-8.
- [CrossRef] [PubMed] [Google Scholar]
- Direct blood pressure-independent anti-fibrotic effects by the selective nonsteroidal mineralocorticoid receptor antagonist finerenone in progressive models of kidney fibrosis. Am J Nephrol. 2021;52:588-601.
- [CrossRef] [PubMed] [Google Scholar]
- Safety and tolerability of the novel non-steroidal mineralocorticoid receptor antagonist BAY 94-8862 in patients with chronic heart failure and mild or moderate chronic kidney disease: A randomized, double-blind trial. Eur Heart J. 2013;34:2453-63.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of finerenone on albuminuria in patients with diabetic nephropathy: A randomized clinical trial. JAMA. 2015;314:884-94.
- [CrossRef] [Google Scholar]
- Effect of finerenone on chronic kidney disease outcomes in Type 2 diabetes. N Engl J Med. 2020;383:2219-29.
- [CrossRef] [PubMed] [Google Scholar]
- Finerenone reduces risk of incident heart failure in patients with chronic kidney disease and Type 2 diabetes: Analyses from the FIGARO-DKD trial. Circulation. 2022;145:437-47.
- [CrossRef] [PubMed] [Google Scholar]
- Design and baseline characteristics of the finerenone in reducing cardiovascular mortality and morbidity in diabetic kidney disease trial. Am J Nephrol. 2019;50:345-56.
- [CrossRef] [PubMed] [Google Scholar]







