Translate this page into:
Effect of Cranberry Extract on Dental Plaque: A Systematic Review

*Corresponding author: Sanpreet Singh Sachdev, Department of Oral Pathology and Microbiology, Bharati Vidyapeeth (Deemed to be University) Dental College and Hospital, Navi Mumbai, Maharashtra, India. sunpreetss@yahoo.in
-
Received: ,
Accepted: ,
How to cite this article: Padawe D, Agrawal A, Takate V, Dighe K, Wankhade AD, Sachdev SS. Effect of Cranberry Extract on Dental Plaque: A Systematic Review. Glob J Med Pharm Biomed Update. 2024;19:11. doi: 10.25259/GJMPBU_12_2024
Abstract
Objectives:
Cranberry extracts have been shown to disrupt the formation of biofilms of oral bacteria, and bacterial adherence. The present review aims to analyze the effect of various forms of cranberry extracts on the composition and quantity of dental plaque. The objectives of the review were to determine whether cranberry extracts can be used as a safe and effective alternative for anti-plaque agents.
Material and Methods:
A systematic search was performed in the following databases: MEDLINE (Ovid), PubMed, PubMed Central, Web of Science Citation Index Expanded (SCIEXPANDED), and Google Scholar using the key terms “Cranberry” AND “Plaque” OR “Biofilm” without any restriction for the time of publication to identify the articles published in the English language. Only in vivo or ex vivo randomized clinical trials were included in the review.
Results:
A total of only seven in vivo studies were found, the earliest of which was conducted in the year 2004. Out of these, three studies were randomized clinical trials, three were in vivo studies, and one study was ex vivo. The data pertaining to the study designs, cranberry extract formulations, and the conclusive findings drawn by the authors are comprehensively summarized in the present review.
Conclusion:
Cranberry has proven to be an effective, safe, and feasible technique for reducing dental plaque as compared to various existing anti-microbial agents such as chlorhexidine. Our review highlights the need for comparing various formulations, concentrations, and methods of delivering the cranberry extracts which can be resolved by further research.
Keywords
Flavonoids
Anti-plaque
Bacterial adhesion
Streptococcus mutans
INTRODUCTION
Dental plaque is the soft, translucent, and tenaciously adherent material that accumulates on the surfaces of teeth. It harbors cariogenic bacteria, cell-free enzymes, polysaccharides, and other host constituents.[1] It is now well-established that the formation of dental plaque is a sine qua non for the initiation of dental caries on the smooth surfaces of teeth.[2] The formation of plaque involves a complex process of interactions between the oral environment and bacteria, which proceed in several stages.[3]
Streptococcus mutans is considered the chief bacterial species responsible for the initiation of dental plaque formation. It has at least two specific virulence traits that are involved in the formation of plaque on the tooth surface: (i) Elaboration of extracellular polysaccharides such as glucans and dextrans through glucosyl transferases (GTFs) and (ii) ability to produce acids as well as thrive in acidic pH.[4-6] S. mutans have developed mechanisms to alleviate the influences of acidification by increasing proton-translocating F-ATPase activity in response to low pH.[6,7] A strong causal relationship between Streptococci (S. mutans and Streptococcus sobrinus), Lactobacilli, and Actinomyces present in the plaque was established with the incidence of caries.[8]
Dental plaque is, thus, known to be more resistant to antimicrobial agents than cells in suspension due to higher biomass densities and decreased metabolic activities in biofilms.[9] By aiming to disrupt the ability of S. mutans to utilize sucrose to form acids and glucans on the tooth surface, therapeutic approaches to reducing the formation of cariogenic biofilms could be precise and selective.[10]
Numerous drugs and drug delivery systems have been tested for their effect on dental biofilm formation and maturation. The most commonly employed systems contain antibacterial agents, which reduce the number of viable microorganisms in the biofilm.[11] At present, chlorhexidine (CHX) is still widely regarded as the standard for chemical control of oral plaque, with various concentrations and formulations being recommended.[12] Nonetheless, using this antimicrobial solution can lead to certain undesirable effects, including disruptions to taste perception, a burning sensation in the mouth, mucosal dryness, and staining of teeth and oral tissues.[13,14] Moreover, there is evidence of CHX’s cytotoxic potential.[15] Thus, despite its efficacy, the use of such antibacterial treatments presents a range of unfavorable consequences.
Innovative approaches aim to modify oral bacterial ecology by manipulating bacterial adhesion within biofilms while preserving bacterial viability. These anti-adhesion strategies encompass techniques involving antibodies, adhesion site analogs, and receptor analogs.[16] These agents focused on anti-adhesion help decrease the overall mass of responsible microorganisms without compromising the survival of oral bacteria. This approach aims to curtail the emergence of resistant strains or secondary infections. Thus, the application of anti-adhesion agents appears to be a promising approach to maintaining good oral hygiene. This approach has an advantage over anti-bacterial agents, as almost no bacterial resistance is encountered, as occurs in the former.[17]
Due to these reasons, natural plant extracts that have anti-adhesive properties are constantly being researched in an attempt to develop safer and more economical alternatives to the existing anti-bacterial and anti-plaque agents.[18]
Cranberry (Vaccinium macrocarpon Ait., Ericaceae) juice and hydroalcoholic crude extracts have been shown to disrupt the formation of biofilms of oral bacteria, and the bacterial adherence on apatitic surfaces in vitro.[10] The extracts from this source exhibit antibacterial properties against a range of oral bacterial species that were assessed, including Streptococcus gordonii, S. sobrinus, Fusobacterium nucleatum, Aggregatibacter actinomycetemcomitans, Porphyromonas gingivalis, and Enterococcus faecalis. Moreover, these effects are observed while having minimal or negligible cytotoxic impact on human gingival fibroblasts.[18]
Earlier research has revealed that cranberry extracts, comprising mixtures of flavonols or proanthocyanidins (PACs), hinder the synthesis of glucans by surface-adsorbed GTFs B and C. In addition, they curtail the acidogenicity of S. mutans within biofilms.[10,19] Another bioactive constituent of cranberry extract is non-dialyzable material (NDM). Mouthwash formulated with NDM has demonstrated a substantial reduction in salivary S. mutans levels and the overall bacterial count.[17] So far, though, only limited information is available that focuses on the anti-plaque activity of extracts derived from cranberry on oral pathogenic bacteria. Therefore, the present review aims to analyze the effect of various forms of cranberry extracts on the composition and quantity of dental plaque. The objectives of the review were to determine whether cranberry extracts can be used as a safe and effective alternative for anti-plaque agents.
MATERIAL AND METHODS
A systematic search was performed in the following databases: MEDLINE (Ovid), PubMed, PubMed Central, Web of Science Citation Index Expanded (SCIEXPANDED), and Google Scholar using the key terms “Cranberry” AND “Plaque” OR “Biofilm.” The search was filtered for the articles published in the English language without any restriction for the time of publication. To maximize the number of results found, multiple databases were selected for searching the articles relevant to our review topic. Furthermore, cross-references of the articles included in the final analysis were also scanned for additional eligible studies that may have been missed during the search. The PICO criteria used for inclusion or exclusion of studies is listed in Table 1. Only in vivo or ex vivo randomized clinical trials were included in the review. Cross-sectional studies, in vitro studies, case-control and cohort studies, case reports, and review articles were excluded from the study.
| Category | Inclusion Criteria | Exclusion Criteria |
|---|---|---|
| Population/Participants | • Apparently healthy individuals without any restriction of age. • The study population can include pediatric and adult patients |
• Studies with subjects having systemic disorders • Studies with subjects having active periodontal pathologies |
| Intervention | • Cranberry extracts in the form of mouthwashes, toothpaste, gels | • Ambiguity regarding the formulation of the intervention used |
| Comparison | • The amount and composition of plaque before the prescription/use of the Cranberry extracts would serve as the control for understanding the effect of cranberry extracts after use for a certain period of time on the same | • Studies not comparing the levels of plaque or S mutans before and after the use of cranberry extracts |
| Outcome | • The conclusions drawn by authors based on Oral Indices such as Gingival or Plaque Index (Loe and Silness) before and after the use of Cranberry extracts would be measured as the main outcome of cranberry extract on the dental plaque. • Changes in the S. mutans colony count or inhibition zones on blood agar would be recorded as additional outcomes. |
• Studies not assessing plaque-related factors |
| Study design | • in-vivo or ex-vivo randomized clinical trials | • Cross-sectional studies • In-vitro studies • Case-control studies • Cohort studies • Case reports and case series • Review articles |
Recorded outcomes
The authors, year of study, design of the study, sample size, age range, and method of randomization used were recorded. Details relevant to the preparation of cranberry extract used by the patients, including the concentration, form of intervention, frequency, and duration of use, were recorded. The conclusions drawn by authors based on oral indices such as gingival or plaque index (Loe and Silness) before and after the use of cranberry extracts were measured as the main outcome of cranberry extract on the dental plaque. Changes in the S. mutans colony count or inhibition zones on blood agar were recorded as additional outcomes.
Risk of bias (ROB) assessment
The methodological quality of the included clinical trials and randomized controlled studies was assessed using the Cochrane Collaboration ROB-2 tool. This tool evaluates several domains, such as random sequence generation (selection bias), allocation concealment (selection bias), blinding of personnel and equipment (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias), and other biases. These assessments were conducted using signaling questions in Review Manager 5.3 software.
Each study’s risk level was categorized as low, moderate, or high based on the evaluation of these domains. A study was deemed to have an overall low risk only if all domains were assessed as low risk. If any of the six domains were rated as high risk, the study was considered to have a high overall risk. Studies were classified as having moderate risk if one or more domains were uncertain, but none were rated as high risk.
RESULTS
A total of seven studies were identified that fulfilled the eligibility criteria of the present systematic review [Figure 1].[17,20-25] Findings from these studies are summarized in Tables 2 and 3.
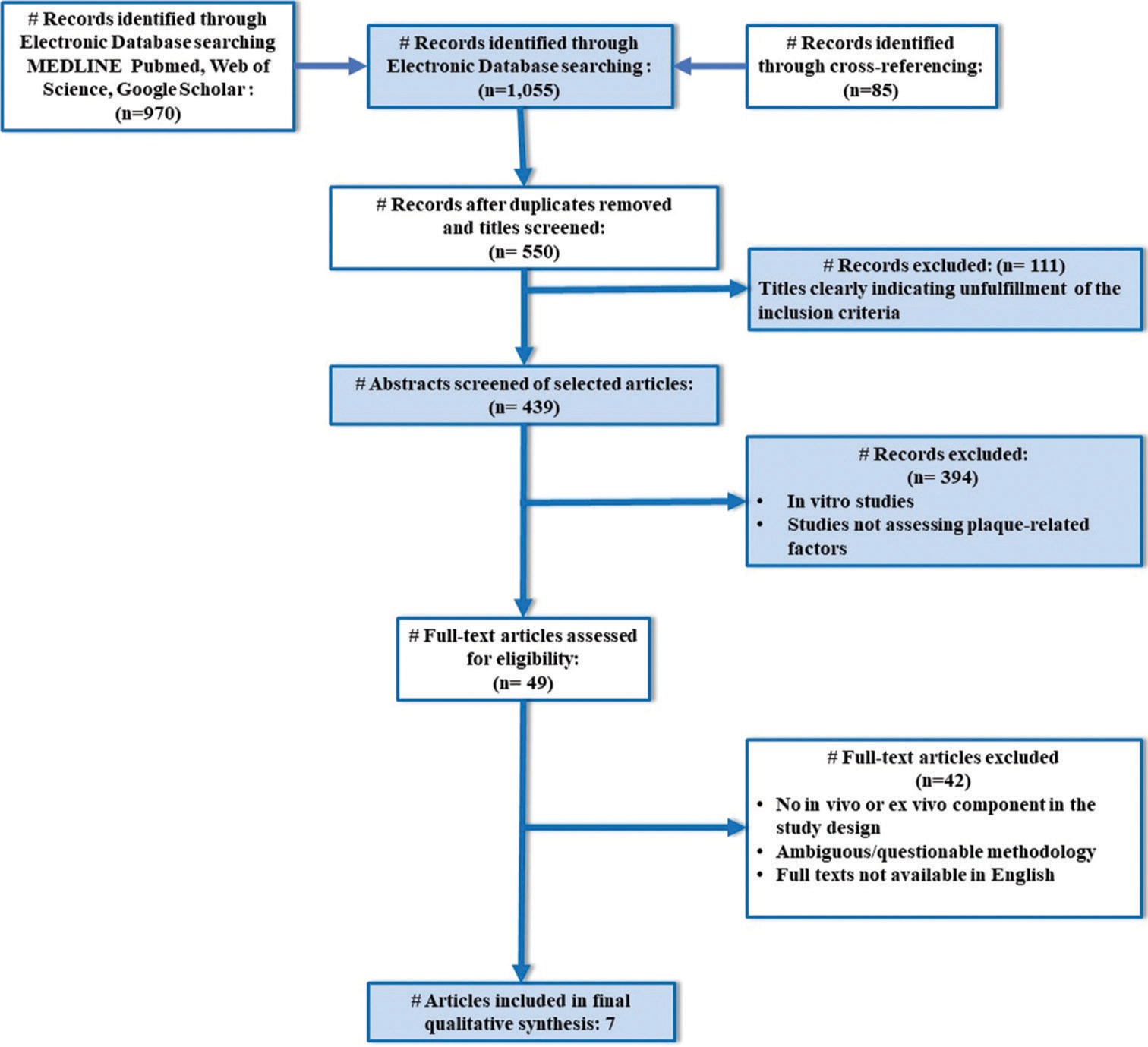
- PRISMA flow diagram indicating the selection process of the articles in the present systematic review, # indicates number or articles.
| Author | Study design | Sample size | Age range (years) |
Inclusion | Exclusion | Randomization |
|---|---|---|---|---|---|---|
| Weiss (2004) |
Parallel-arm design Double-blinded RCT | 60 | 21 to 39 | Healthy volunteers | (i) Antibiotic treatment within 1 month prior to the study (ii) Oral soft tissue pathology other than marginal gingivitis (iii) Missing teeth (iv) Untreated visible carious lesions (v) Smoking |
Performed, Method NS |
| Sethi and Govila (2011) | Ex Vivo Study | 10 | 20 to 30 | Not specified | Not specified | Not specified |
| Gupta et al. (2015) | Parallel-arm design Single-blinded RCT | 40 | 9 to 12 | At least four decayed and/or missing due to caries or filled teeth | (i) History of current or recent (at least for the past 1 month) antibiotic usage. (ii) Abscess, draining sinus, cellulitis, or other conditions requiring emergency dental treatment. |
Performed, Method NS |
| Khairnar et al. (2015) | Parallel-arm design Double-blinded RCT | 50 | 18-20 | (i) Subjects with good general health (ii) Agreement to delay any elective dental treatment including oral prophylaxis (iii) Agreement to comply with the study visits were included in the study. |
(i) Subjects with severe mal-alignment of teeth (ii) Orthodontic appliances (iii) Fully crowned teeth (iv) Removable partial dentures; (v) Subjects already using mouthwash or dental floss (vi) Tobacco consumers, (vii) Medical or pharmacological history |
Lottery method |
| Woźniewicz et al. (2018) | Parallel-arm design RCT | 50 | 16-55 | (i) Patients with gingivitis (ii) Good general health (iii) Aged 16–55 years |
(i) Systemic diseases such as diabetes mellitus, and autoimmune diseases (ii) Antibiotic therapy in the last 3 months (iii) Current pregnancy or breastfeeding, (iv) Implants, and dentures, (v) Food allergies to any compounds contained in the interventional product |
Blocked randomization by computer software |
| Philip et al. (2019) | Parallel-arm design double-blinded RCT |
90 | >10 | (i) Minimum of 10 years of age (ii) At least 4 fully erupted permanent maxillary teeth undergoing fixed orthodontic treatment in both arches with treatment having been underway for at least 1 month (iii) Not currently using antibiotics/antimicrobial mouth rinses (iv) Available to attend a recall appointment in 5 to 6 weeks |
(i) Any medical condition or disability preventing self-tooth brushing (ii) allergy to milk casein proteins or benzoate preservatives present in the CPP-ACP toothpaste (iii) Unwillingness to use a fluoridated toothpaste, (iv) Untreated periodontal disease or clinical evidence of active caries. |
Random number generation function |
| Luu (2020) | Crossover design RCT | 15 | 7 to 12 | (i) 7-12 years of age (ii) Good general and gingival health (iii) No allergies or opposition to any of the beverages to be tested (iv) Willing to participate in up to four 30-45 minute appointments |
(i) <7 or>12 years of age (ii) Any systemic disease (such as asthma, diabetes) (iii) Clinical evidence of spontaneous gingival bleeding (iv) History of irritation or sensitivity to the test beverages (v) Not willing to give written assent to participate (vi) Subjects taking any antimicrobial medication therapy |
Performed, Method NS |
| Author | Concentration used | Form used | Frequency | Duration of use | Indices used | Tests for bacteria | Conclusive findings |
|---|---|---|---|---|---|---|---|
| Weiss (2004) | 3 mg/ml | Mouthwash | 15 ml twice a week | 6 weeks | (i) Turesky modification of the Quigley & Hein plaque index (ii) Loe & Silness GI |
(i) Unstimulated saliva on culturing device using trypticase for total bacterial count (ii) Mitis salivarius agar for S mutans count (iii) S mutans adhesion test son hydroxyapatite beads |
Continuous use of mouthwashes containing NDM derived from cranberries are safe, edible and non-fermentable anti-adhesion agents that represent a novel approach to altering biofilm formation on teeth, |
| Sethi and Govila (2011) | 6mg/ml | Gel | Unclear | Unclear | NA | Culture of plaque samples on blood agar plate for streptococcus species using Lance Field Test | Cranberry gel in highly concentrated (1:600) form has an inhibitory effect on the colonization of the Streptococci species, and thus can be beneficial in the inhibition of dental plaque formation. |
| Gupta et al. (2015) | 3mg/ml | Mouthwash | 5ml once daily | NA | NA | S. mutans levels in plaque using Dentocult SM strips | The high-molecular-weight cranberry extract is highly efficacious in reducing the S. mutans count in the oral environment |
| Khairnar et al. (2015) | 6mg/ml | Mouthwash | 10ml twice daily | 14 days | NA | Innoculation in blood agar and counting S mutans colonies by digital colony counter | Cranberry mouthwash is equally effective as Chlorhexidine mouthwash with beneficial local and systemic effect. |
| Woźniewicz et al. (2018) | 20v/v% | Juice | 250ml thrice a day | NA | (i) Loe & Silness GI and PI (ii) Bleeding on probing (iii) Serum & Saliva TAS & MDA (iv) Serum IL-Beta |
CFU counts of S mutans & Lactobacillus | (i) Cranberry juice improves gingival inflammation indices but does not affect bleeding on probing. (ii) It also reduces the counts of S. mutans and lactobacillus. |
| Philip et al. (2019) | 0.25% w/w | Toothpaste | twice daily | 5 to 6 week | NA | Plaque samples tested for 8 caries-associated bacterial species and 6 health-associated commensal bacterial species by real-time quantitative polymerase chain reaction analysis |
Dentifrices containing CPP-ACP and polyphenol-rich cranberry extracts can influence a species-level shift in the ecology of the oral microbiome, resulting in a microbial community less associated with dental caries |
| Luu (2020) | 100% and 27% | Juice | 30ml thrice daily | NA | Plaque glycolysis regrowth method | (i) Consumption of 100% cranberry juice showed significant inhibition of plaque regrowth and acid production. (ii) Cranberry can be considered a “functional food for oral health.” |
Assessment of methodological quality and ROB
The methodological quality of all the included studies was generally comparable, with a moderate to high ROB across all domains. The greatest ROB was associated with allocation concealment (selection bias). Among the studies, Sethi and Govila and Weiss et al. exhibited a higher ROB compared to the others.[17,20] Conversely, Khairnar et al. and Gupta et al. showed the lowest ROB.[15,22] The studies indicated the lowest ROB in domains related to selective reporting (reporting bias) and other biases. The highest ROB was observed in allocation concealment, followed by random sequence generation (selection bias), blinding of personnel and participants (performance bias), and blinding of outcome assessment (detection bias). The ROB in included studies is depicted in Figures 2 and 3.
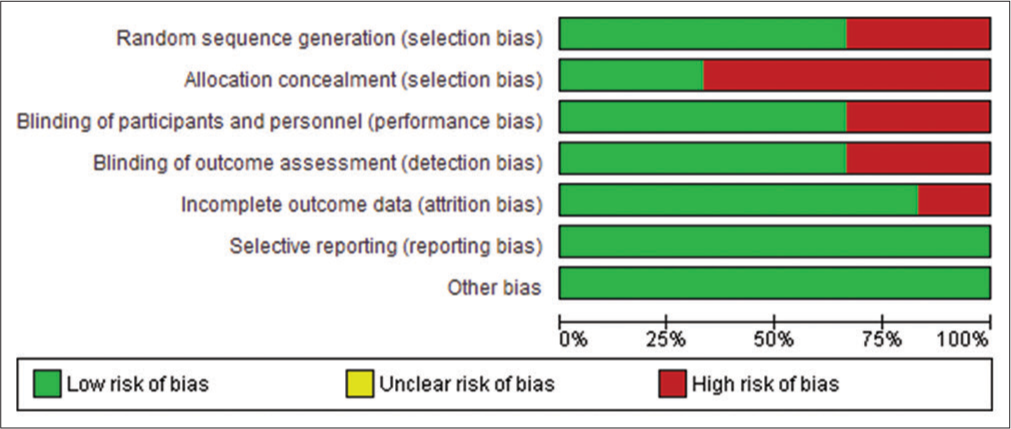
- Risk of bias (ROB) graph: Review authors’ judgments about each ROB item presented as percentages across all included studies. The scale is a standard format for risk of bias, which has inherent green, yellow and red components. The degree of risk of bias may vary across studies and may or may not include all the three categories.

- Risk of bias (ROB) summary: Review authors’ judgments about each ROB item for each included study. Green: Low risk of bias, Red: Serious risk of bias
DISCUSSION
A total of only seven in vivo studies were found the earliest of which was conducted in 2004. This indicates that the effect of cranberry extract on dental plaque directly in the oral environment has seldom been studied and requires further exploration. Out of these, three studies were randomized clinical trials, three studies were in vivo studies, and one study was ex vivo. In vitro study designs assessing the impact of cranberry components on various species of oral microflora, although they provide substantial results, cannot completely simulate the oral environment. The oral cavity comprises saliva, which is composed of numerous proteins, enzymes, bacteria, and tissue components.[26] Thus, studying the effect of any anti-plaque agent in vivo would provide practically more realistic information regarding its effectiveness. Thus, only in vivo studies were included in the present systematic review.
Besides these known confounding factors, numerous other factors can influence the subjective and objective assessment of plaque levels. Therefore, it is important that both groups should have similar baseline characteristics of their respective populations. Various randomization techniques have been employed in clinical trials to effectively minimize the skewing of demographic characteristics of the different groups in a study. Randomization eliminates the selection bias and balances the groups with respect to many known and unknown confounding or prognostic variables.[27] The studies included in the present systematic review performed randomization, except for one which did not mention the method of randomization.[17]
The sample sizes of the included studies ranged from 10 to 90, with most of the studies utilizing sample sizes in the range of 50–60. While these sample sizes are acceptable, a larger sample size would definitely be crucial for further increasing the validity and reliability of the results. The age range of the samples used by various authors was 7 to 55 years. An important advantage of analyzing results from studies with a wide range of age groups is that it allows the reviewers to determine the effect of cranberry on plaque in deciduous as well as permanent teeth. The sample population comprised children aged 7 to 12 years in n = 2 studies, while the others mostly included young adults in their samples.[21,25]
The form and concentration of the extract used influences the effectiveness of the final product. The cranberry extracts were used in various forms across the studies included in the present systematic review. The most common form used was mouthwash (n = 3), followed by juice (n = 2), gel (n = 1), and toothpaste (n = 1). There were no undesirable effects of the mouthwash observed in any of the included studies. The mouthwash was palatable, did not cause any staining, and was well-accepted by the samples. The advantage of using cranberry extract mouthwash is that it is an herbal agent, so there are no chances of toxicity. Second, since it does not affect the viability of oral bacteria, bacterial resistance will not develop. Third, it does not cause any staining of the teeth. The main disadvantage is that it is not readily available in the market. Moreover, the procedure involved in the extraction and preparation of mouthwash from cranberry extracts is expensive and requires laboratory setup.[18,21]
Results from an investigation carried out by Sethi and Govila unveiled that cranberry gel in a highly concentrated form (1:600) exhibits the ability to hinder the colonization of Streptococci species. This characteristic could have advantageous implications in thwarting the development of dental plaque.[17] Moreover, NDM in cranberry extracts influences the phosphorylation and expression of various intracellular proteins linked to matrix metalloproteinase (MMP) production. Consequently, it presents potential as an anti-biofilm agent. The NDM fraction of cranberry might notably operate by suppressing activator protein-1 activity, consequently inhibiting MMP production.[28] As a result, the authors suggested the utilization of concentrated cranberry juice or mouthwashes as a preventive measure against biofilm formation. The merit of multiple forms of the extract is that the effectiveness of using the extract in specific formulations can be determined. However, no study has yet directly compared the effectiveness of two formulations. Therefore, future studies are encouraged to compare the effectiveness of two or more different forms of cranberry extracts.
The concentrations of 3 mg/mL and 6 mg/mL were commonly employed in two studies each, respectively.[17,20-22] Concentrations of 20% v/v and 0.25% w/v were also used in one study Luu compared the effectiveness of commercially available 27% and 100% concentrations of cranberry juices.[25] The variation in the concentrations of extracts used across all the studies implies that there is yet no standardization regarding the posology of Cranberry extracts to be used for optimal effectiveness. Care must be taken to not exceed concentrations beyond which the intervention may pose harm to the delicate oral mucosa. In a comparative study concerning various berry juices, only freshly prepared highly concentrated blackcurrant juice was found to have slight or only very limited cytotoxic effects on human gingival fibroblasts, whereas cranberry extract has been found to be safe to use even at higher concentrations.[18]
Besides the form and concentration of extracts, the frequency of use also plays an important role in reinforcing the effect of the formulations in the oral cavity. Most of the studies (n = 3) that used mouthwash recommended rinsing with 10–15 mL twice daily and 5 mL once daily. Luu advised the consumption of 30 mL of juice thrice daily in either 100% or 27% concentrations[25] whereas Woźniewicz et al. recommended the consumption of 250 mL of juice thrice a day.[23] Philip et al.[24] recommended brushing teeth twice daily, as done with any other toothpaste. However, in the study conducted by Sethi and Govila, which used gel form, a definite frequency of usage was not clearly defined. A duration of use ranging from two to six weeks was recommended across the various studies included in the review.
The gingival and plaque indices provide clinically relevant information regarding plaque levels.[29-31] Significant alterations in these indices would indicate the effectiveness of the intervention. The gingival and plaque indices devised by Loe and Silness are the most common indices used by investigators.[29,30] Two studies used Loe and Silness gingival and plaque index, and one study used Turesky modification of the Quigley and Hein plaque index and plaque glycolysis regrowth method.[25,31]
Objective assessment of the plaque composition is performed by counting the colonies of S mutans. This was performed by either culturing or by means of digital colony counters. Blood agar was most commonly employed since coupled with the Lancefield test, it effectively indicates the type of hemolysis caused by the corresponding species of streptococci.[32] Another agar used was mitis salivarius agar. In the study conducted by Philip et al., the plaque samples were tested for eight caries-associated bacterial species and six health-associated commensal bacterial species by real-time quantitative polymerase chain reaction analysis.[24] PCR offers the advantage of producing highly specific and sensitive results even with a minimum amount of samples.[33] The only drawback of this method is the equipment’s feasibility, cost, and maintenance.
The review’s various studies generally found Cranberry extracts to be effective in reducing dental plaque. Cranberry juice has been shown to prevent some bacteria from adhering to surfaces. Weiss’s et al. study concluded that NDM does not affect the viability of mutant streptococci, including S. sobrinus, or other plaque bacteria.[20] The observed reduction in S. mutans was attributed to the compound’s anti-adhesion properties. Gupta et al. found that Cranberry extract significantly reduces S. mutans counts in the oral cavity.[21] Khairnar et al. compared cranberry mouthwash to CHX mouthwash, finding both equally effective, suggesting cranberry mouthwash as a viable alternative to antimicrobial options like CHX.[22]
Notably, S. sobrinus, F. nucleatum, and P. gingivalis are particularly susceptible to cranberry juice treatment. Duarte et al. identified several mechanisms by which flavonols and PACs in cranberries could impact S. mutans virulence: Inhibiting insoluble glucan synthesis by surface-bound GTF B and C, disrupting proton-translocating F-ATPase activity, and interfering with acid production.[19] While these findings show the potential of cranberry components to hinder S. mutans virulence, the exact active compound(s) need further investigation. Sethi and Govila’s study found that cranberry juice reduces the cell surface hydrophobicity of certain oral streptococci, correlating with juice concentration, suggesting that cranberry juice components may bind to or mask hydrophobic proteins on streptococci cell surfaces.[17]
Woźniewicz’s et al. research concluded that cranberry juice mitigates gingival inflammation indices but does not notably impact bleeding on probing.[23] However, it does lead to a reduction in oral bacterial load. Philip’s et al. research showed that dentifrices containing casein phosphopeptides-amorphous calcium phosphate (CPP-ACP) and cranberry extracts rich in polyphenols can alter the oral microbiome at the species level, leading to a microbial environment less prone to dental caries.[24] Luu’s work during his dental studies demonstrated that consuming 100% cranberry juice significantly inhibits plaque regrowth and acid production. He asserted that cranberry deserves recognition as a “functional food for oral health.”[25]
The high-molecular-weight NDM in cranberry juice, known for its tannin-like properties and high water solubility, acts as an active ingredient. It effectively disrupts the co-aggregation of most bacterial pairs, lacking proteins, carbohydrates, and fatty acids, and contains 56.6% carbon and 4.14% hydrogen.[18,20] Studies have demonstrated that pre-coating bacteria with NDM reduces their biofilm-forming ability, consistent with findings in this systematic review.
A notable limitation of the present systematic review is the small number of in vivo studies available, with only seven studies included, dating back to 2004. This limited number of studies restricts the breadth and depth of the analysis, potentially affecting the generalizability of the findings. Furthermore, the variability in study designs, sample sizes, age groups, and concentrations of cranberry extracts used adds heterogeneity to the results, making it challenging to draw definitive conclusions. The absence of direct comparative studies between different forms of cranberry extracts further limits the ability to recommend the most effective formulation. In addition, the high ROB in some studies, particularly regarding allocation concealment and randomization methods, raises concerns about the reliability of the findings. Finally, the lack of standardization in the posology of cranberry extracts necessitates cautious interpretation of the results, highlighting the need for more rigorous and standardized future research.
Future research should focus on conducting more extensive in vivo studies with larger and more diverse sample sizes to enhance the generalizability of findings, while also developing standardized protocols for the concentration and formulation of cranberry extracts to determine the optimal posology for dental plaque prevention. Comparative studies between different forms of cranberry extracts, such as mouthwash, juice, gel, and toothpaste, are needed to identify the most effective formulation. In addition, long-term efficacy and safety studies, including potential side effects, are crucial to ensure cranberry extracts are a viable alternative to traditional antimicrobial agents. Mechanistic studies should explore how cranberry extracts, particularly the NDM, affect the oral microbiome and inhibit biofilm formation at the molecular level, focusing on key oral bacteria such as S. mutans, S. sobrinus, F. nucleatum, and P. gingivalis. Finally, clinical trials with a rigorous methodology that ensures proper randomization, blinding, and allocation concealment are essential to validate the effectiveness and reliability of cranberry extracts in oral health.
CONCLUSION
Cranberry has proven to be an effective, safe, and feasible technique for reducing dental plaque as compared to various existing anti-microbial agents such as CHX. While a multitude of cranberry formulations have been used to combat dental plaque, the issue of standardization of form and concentration of the extracts is yet to be addressed. The present review highlights the need for comparing these formulations, concentrations, and methods of delivering the cranberry extracts which can be resolved by further research.
The findings from this systematic review underscore the potential of cranberry extracts as effective agents in reducing dental plaque and improving oral health. The demonstrated efficacy of cranberry-based mouthwashes, gels, and other formulations in inhibiting bacterial adhesion and biofilm formation suggests a promising alternative to traditional antimicrobial products, potentially enhancing patient outcomes by offering a natural, non-toxic option that does not contribute to bacterial resistance.
However, to fully integrate cranberry extracts into clinical practice, future research should focus on conducting larger, more diverse in vivo studies, developing standardized protocols for extract concentrations and formulations, and performing direct comparative studies to identify the most effective forms. In addition, long-term safety and efficacy trials are needed to ensure that these products can be used safely over extended periods. Mechanistic studies to elucidate the molecular pathways through which cranberry extracts exert their effects, particularly on key oral bacteria, will further solidify their role in dental care. Rigorous clinical trials with proper randomization, blinding, and allocation concealment will be essential to validate these findings and support the broader adoption of cranberry extracts in clinical settings.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
Patient consent was not required as there are no patients in this study.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript, and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Extracellular Polysaccharides Matrix-An Often Forgotten Virulence Factor in Oral Biofilm Research. Int J Oral Sci. 2009;1:229.
- [CrossRef] [Google Scholar]
- Oral Biofilm and Its Impact on Oral Health, Psychological and Social Interaction. Int J Oral Dent Health. 2021;7:127-37.
- [CrossRef] [Google Scholar]
- Streptococcus mutans-Derived Extracellular Matrix in Cariogenic Oral Biofilms. Front Cell Infect Microbiol. 2015;5:10.
- [CrossRef] [Google Scholar]
- Biology of Streptococcus mutans-derived Glucosyltransferases: Role in Extracellular Matrix Formation of Cariogenic Biofilms. Caries Res. 2011;45:69-86.
- [CrossRef] [Google Scholar]
- Acid-adaptive Mechanisms of Streptococcus mutans-the More We Know, the More We Don't. Mol Oral Microbiol. 2017;32:107.
- [CrossRef] [Google Scholar]
- The F-ATPase Operon Promoter of Streptococcus mutans is Transcriptionally Regulated in Response to External pH. J Bacteriol. 2004;186:8524-8.
- [CrossRef] [Google Scholar]
- Dental Caries: The Disease and Its Clinical Management (2nd ed). Oxford, UK: Blakswell Munksgaard; 2008.
- [Google Scholar]
- Dynamics of Dissolution, Killing, and Inhibition of Dental Plaque Biofilm. Front Microbiol. 2020;11:518834.
- [CrossRef] [Google Scholar]
- Influence of Cranberry Phenolics on Glucan Synthesis by Glucosyltransferases and Streptococcus mutans Acidogenicity. J Appl Microbiol. 2007;103:1960-8.
- [CrossRef] [Google Scholar]
- Emerging Applications of Drug Delivery Systems in Oral Infectious Diseases Prevention and Treatment. Molecules. 2020;25:516.
- [CrossRef] [Google Scholar]
- Chlorhexidine Mouthrinse as an Adjunctive Treatment for Gingival Health. Cochrane Database Syst Rev. 2017;3:CD008676.
- [CrossRef] [Google Scholar]
- Chlorhexidine (CHX) in Dentistry: State of the Art. Minerva Stomatol. 2012;61:399-419.
- [Google Scholar]
- The Effect of an Oxygenating Agent on Chlorhexidine-induced Extrinsic Tooth Staining: A Systematic Review. Int J Dent Hyg. 2012;10:198-208.
- [CrossRef] [Google Scholar]
- Effect of Chlorhexidine Digluconate on Different Cell Types: A Molecular and Ultrastructural Investigation. Toxicol In Vitro. 2008;22:308-17.
- [CrossRef] [Google Scholar]
- Anti-adhesion Therapy of Bacterial Diseases: Prospects and Problems. FEMS Immunol Med Microbiol. 2003;38:181-91.
- [CrossRef] [Google Scholar]
- Inhibitory Effect of Cranberry Juice on the Colonization of Streptococci Species: An In Vitro Study. J Indian Soc Periodontol. 2011;15:46-50.
- [CrossRef] [Google Scholar]
- Antimicrobial Effect of Natural Berry Juices on Common Oral Pathogenic Bacteria. Antibiotics (Basel). 2020;9:533.
- [CrossRef] [Google Scholar]
- Inhibitory Effects of Cranberry Polyphenols on Formation and Acidogenicity of Streptococcus mutans Biofilms. FEMS Microbiol Lett. 2006;257:50-6.
- [CrossRef] [Google Scholar]
- A High Molecular Mass Cranberry Constituent Reduces Mutans Streptococci Level in Saliva and Inhibits In Vitro Adhesion to Hydroxyapatite. FEMS Microbiol Lett. 2004;232:89-92.
- [CrossRef] [Google Scholar]
- Effect of High-molecular-weight Component of Cranberry on Plaque and Salivary Streptococcus mutans Counts in Children: An In Vivo Study. J Indian Soc Pedod Prev Dent. 2015;33:128-33.
- [CrossRef] [Google Scholar]
- Comparative Assessment of Cranberry and Chlorhexidine Mouthwash on Streptococcal Colonization among Dental Students: A Randomized Parallel Clinical Trial. Contemp Clin Dent. 2015;6:35.
- [CrossRef] [Google Scholar]
- Consumption of Cranberry Functional Beverage Reduces Gingival Index and Plaque Index in Patients with Gingivitis. Nutr Res. 2018;14:36-45.
- [CrossRef] [Google Scholar]
- Randomized Controlled Study to Evaluate Microbial Ecological Effects of CPP-ACP and Cranberry on Dental Plaque. JDR Clin Trans Res. 2020;5:118-26.
- [CrossRef] [Google Scholar]
- Effect of Cranberry Juice on Dental Plaque in Children (Doctoral Dissertation. University of Illinois at Chicago).
- [Google Scholar]
- Characterisation of Human Saliva as a Platform for Oral Dissolution Medium Development. Eur J Pharm Biopharm. 2015;91:16-24.
- [CrossRef] [Google Scholar]
- A Roadmap to Using Randomization in Clinical Trials. BMC Med Res Methodol. 2021;21:168.
- [CrossRef] [Google Scholar]
- Inhibition of Streptococcus mutans Adsorption to Hydroxyapatite Bylow-molecular Weight Chitosans. J Dent Res. 1997;76:665-72.
- [CrossRef] [Google Scholar]
- The Gingival Index, the Plaque Index and the Retention Index Systems. J Periodontol. 1967;38:610-6.
- [CrossRef] [Google Scholar]
- The Plaque Control Index: A Practical Method of Assessing the Effectiveness of Oral Hygiene Procedures. J DASA. 1977;32:397-9.
- [Google Scholar]
- A Four-week Clinical Study to Evaluate and Compare the Effectiveness of a Baking Soda Dentifrice and an Antimicrobial Dentifrice in Reducing Plaque. J Clin Dent. 2008;19:120-6.
- [Google Scholar]
- Laboratory Diagnosis of Streptococcus pyogenes (Group A Streptococci) In: Streptococcus pyogenes: Basic Biology to Clinical Manifestations. Oklahoma City: University of Oklahoma Health Sciences Center; 2016.
- [Google Scholar]
- Real-time Quantitative Polymerase Chain Reaction for Enumeration of Streptococcus mutans from Oral Samples. Eur J Oral Sci. 2011;119:447-54.
- [CrossRef] [Google Scholar]







