Translate this page into:
Comparison of Remineralization Potential of Theobromine and Nano-Hydroxyapatite to Sodium Monofluorophosphate: An In Vitro Study

*Corresponding author: Faiz Ansari, Department of Pediatric and Preventive Dentistry, Bharati Vidyapeeth (deemed to be university) Dental College and Hospital, Pune, India. dransarifaiz@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Ansari F, Chaudhary SM, Jajoo S, Lakade LS, Dhavalbhakta R, Kunthe S, et al. Comparison of Remineralization Potential of Theobromine and Nano-Hydroxyapatite to Sodium Monofluorophosphate: An In Vitro Study. Glob J Med Pharm Biomed Update. 2025;20:6. doi: 10.25259/GJMPBU_6_2024
Abstract
Objectives
The present in vitro study evaluated and compared the effect of theobromine gel and nanohydroxyapatite (NHA) paste to conventional sodium monofluorophosphate-containing toothpaste on demineralized enamel. The study’s objectives were to investigate the remineralizing potential and effect of the materials on the microhardness and surface roughness (SR) of demineralized enamel.
Material and Methods
The present in vitro study was conducted in Bharati Vidyapeeth (Deemed to be) Dental College and Hospital, Pune. The laboratory imaging and analysis were performed at Praj Metallurgical laboratory, Pune, over one month from December 2022 to January 2023. A total of 30 (n = 30) extracted human premolars were decoronated and embedded in customized acrylic resin molds. A window of 5 × 5 mm on the buccal surface was demineralized for three days and then subjected to a 21-day pH cycle, following which it was remineralized. Surface microhardness (SMH) and SR were recorded at baseline, after demineralization and remineralization by respective agents (n = 10 samples each). Scanning electron microscopy was also performed for the samples.
Results
A significant increase (P < 0.05) was observed in the SMH values in all three groups, although none of the groups reached the original microhardness values that were present at the baseline. The SMH values were highest in the sodium fluoride group after remineralization while the lowest increase was noted with theobromine. The specimens treated with NHA exhibited the highest sedimentation of the porosities and the least SR under scanning electron microscopy.
Conclusion
Sodium monofluorophosphate, theobromine, and NHA are effective remineralizing agents. While sodium monofluorophosphate achieved the highest microhardness increase, its improvement in SR was not at par with the other two materials. NHA excelled in improving the SMH and roughness.
Keywords
Dental caries
Electron
Microscopy
Scanning
Sodium fluoride
Theobromine
Toothpastes
INTRODUCTION
The classic definition of dental caries states that it is “an irreversible microbial disease of the calcified tissues of the teeth, characterized by demineralization of the inorganic portion and destruction of the organic substance of the tooth, which often leads to cavitation.”[1] The inorganic portion of tooth structure in both enamel and dentin is made up of calcium hydroxyapatite (CHA) crystals. With the evolution of knowledge concerning the pathogenesis of caries, it was understood that the disease is not merely a unidirectional process of continuous demineralization but also involves intermittent periods of remineralization.[2]
This remineralization can be attributed to the exposure of the tooth’s outermost surface, whether decayed or intact, to the saliva and plaque fluids that are super-saturated with calcium and phosphates. Through these conditions encouraging remineralization, the caries progression can be retarded or even arrested. The addition of fluoride or water to the diet has been demonstrated to reduce the prevalence of dental caries due to the formation of calcium fluorapatite (CFA) crystals.[3] These CFA crystals are even more resistant to acid disintegration than the naturally existing CHA.[1] Therefore, dentifrices containing fluoride in the form of sodium fluoride or sodium monofluorophosphate are popularly used today.
However, fluoride is tolerable only to a certain dosage within the body, and the harmful effects of greater concentrations of fluoride cannot be overlooked. Concentrations of fluoride exceeding one ppm begin manifesting clinically as hypoplastic areas on teeth in the form of dark pits, stripes, or a corroded appearance of the crown. Higher dosages can also cause systemic effects such as early aging, decreased intelligence, and irritation of the gastrointestinal tract.[4,5] Due to these adverse effects, modern-day manufacturers have attempted to formulate fluoride-free dental healthcare products that aim to remineralize teeth.
Theobromine, with the chemical formula 3.7 dimethylxanthine, is a white crystal powder that has been used to prevent enamel demineralization.[6] It causes calcium and phosphate from the saliva to merge into a crystal unit that is four times bigger than hydroxyapatite, causing regrowth of enamel crystals. The combination of mineral placement as new enamel growth takes place increases the enamel hardness.[7] Although gels and dentifrices containing theobromine have already been developed and marketed, they have yet to gain popularity.
Nano-hydroxyapatite (NHA), a biomimetic material, can reconstruct hypomineralized enamel. Dentifrices consisting of NHA were first introduced in the 1980s and were approved by the Japanese government as anti-caries agents in 1993.[8] It has been the subject of various studies; however, most of these studies were conducted at the manufacturer’s request, and the outcomes were primarily disseminated in Japanese journals.[9] Studies describing the remineralization benefits of NHA-containing toothpaste are scarce, particularly in the Indian context.
Despite the advancements in dental care products, there is a significant gap in the literature regarding the comparative effectiveness of emerging fluoride-free alternatives such as theobromine gel and NHA paste against conventional fluoride-containing toothpaste, specifically in the context of the Indian population. Most studies on NHA have been limited in scope or sample size, making it difficult to generalize their findings globally. In addition, while theobromine-based products have shown promise in enhancing enamel hardness, their acceptance and widespread use remain limited due to a lack of comprehensive, comparative studies.
Hence, the present in vitro study evaluated and compared the effect of theobromine gel and NHA paste to that of conventional sodium monofluorophosphate-containing toothpaste on demineralized enamel. The study aims to investigate these materials’ remineralizing potential and the effect on the microhardness and surface roughness (SR) of demineralized enamel. By addressing this research gap, the study seeks to provide more precise insights into the efficacy of these fluoride-free alternatives, potentially guiding better dental care practices and product development.
MATERIAL AND METHODS
The present in vitro study was conducted in Bharati Vidyapeeth (to be deemed) Dental College and Hospital, Pune, and its laboratory analysis was done at Praj Metallurgical Laboratory, Pune. The study commenced around December 2020 and continued for a month until January 2021. The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Institutional Ethical Review Board (BVDU/DCH/620-1/2020-2021).
Sample size calculation
The sample size for this study was calculated using GPower software to ensure sufficient power to detect statistically significant differences between the groups. The effect size was estimated using data from a previous study conducted by Yuanita et al. (2020).[10] For a two-tailed test comparing the means of two independent groups, with an effect size DDD of 1.9624797, an α\alphaα error probability of 0.05, a power (1-β\betaβ error probability) of 0.95, and an allocation ratio of 1, the required sample size per group was determined to be 10. This calculation yielded a non-centrality parameter δ of 4.3882380, a critical t-value of 2.1009220, and 18 degrees of freedom, resulting in an actual power of 0.9555191. Since the study involves three groups (theobromine gel, NHA paste, and conventional sodium monofluorophosphate-containing toothpaste), the total sample size was set to 30.
Sample preparation
A total of 30 structurally sound human maxillary or mandibular premolars extracted for orthodontic purposes were utilized for the present study. Teeth with incipient white spot lesions, visible caries, restorations, hypoplasia, or cracks were excluded from the study. Teeth with stains or calculus buildup were also excluded from the study.
The samples were disinfected and stored in fresh, renewed, deionized water till the beginning of the experimental procedures. The teeth were decoronated by moving a diamond disc through the cervical region in a buccolingual direction [Figure 1a]. Copious cooling by a continuous jet of cold water was performed to avoid excessive heat generation that would cause damage to the tooth structure.
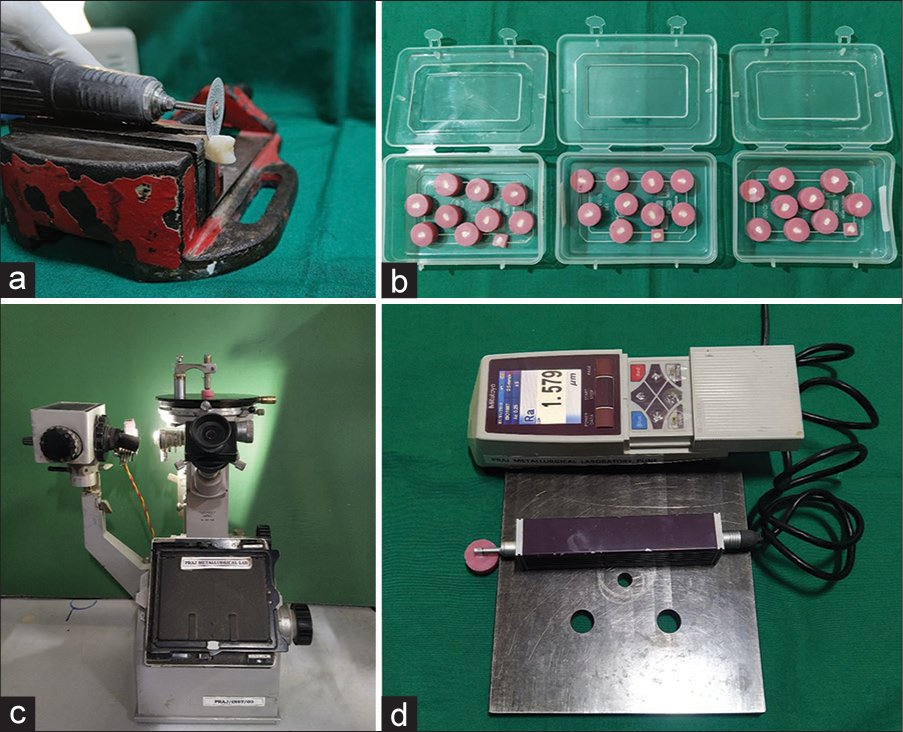
- (a) De-coronation of teeth; (b) prepared samples embedded in resin; (c) microhardness tester; and (d) stylus profilometer.
The teeth were embedded in self-cured acrylic resin poured into customized plastic molds [Figure 1b]. It was ensured that the buccal surface of the tooth was facing upwards which was then subjected to sequential flattening and polishing by 400, 800, 1000, and 1200 grit abrasive papers. All the remaining surfaces of the acrylic blocks were painted with two coats of acid-resistant nail varnish.
On the buccal surface, an adhesive tape of 5 mm × 5 mm dimensions was applied to the central areas while a uniform coat of nail varnish was applied to the remainder of the buccal surface to render it resistant to acid demineralization. After thoroughly drying the samples, the adhesive tape was removed to expose a window of enamel surface susceptible to demineralization by acids.
Outcome recording
The surface microhardness (SMH) of the samples was tested by a Microhardness Tester (Reichert Austria Make, Sr.No.363798, Load: 50 g, Reference Standard: ISO 6507); [Figure 1c]. The SR measurement was carried out by Stylus profilometer [Figure 1d], Mitutoyo, Japan. Model: SJ 210. Scanning electron microscopic (SEM) images were also obtained for the specimens.
Preparation of solutions
The solutions were prepared as described in an earlier study.[11] The demineralizing solution was prepared with 50 mM acetic acid (pH 4.5), 2.2 mM potassium dihydrogen phosphate (KH2 PO4), 2.2 mM calcium nitrate, and 0.1 ppm sodium fluoride. The remineralization solution used in pH-cycling contained 20 mMol l−1 HEPES(4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid), 130 mM potassium chloride, 1.5 mM calcium chloride, 0.9 mM KH2 PO4, and 1 mM sodium azide. The pH was adjusted to 7.0 with potassium hydroxide.
Demineralization procedure
The samples were then immersed individually in 20 mL demineralizing solution (pH 4.4) for 96 h to develop incipient carious lesions in the enamel characterized by demineralization and SR. The SMH and SR values were recorded again along with SEM imaging, following which a 21-day pH cycling model was commenced to simulate environmental conditions of the oral cavity.
Remineralization-demineralization pH cycling model
The samples were equally divided into three groups comprising n = 10 samples each, which included Group A (Sodium fluoride paste), Group B (Theobromine gel), and Group C (NHA paste). The respective remineralizing agents were then applied to the samples with applicator tips. After two minutes, the samples were cleaned with deionized water and then immersed in 20 mL of demineralizing solution for three hours. After thorough washing with deionized water, the remineralizing agents were again applied for two minutes. The samples were then submerged in 20 mL of the remineralizing solutions (pH 7) for 17 h. The solutions were replaced every 48 hours, and these procedures were continued cyclically for 21 days. After remineralization, the final SMH and SR recording was done after the completion of the 21-day pH cycling model, along with SEM imaging of the samples.
Statistical analysis
The data were recorded in a Microsoft Excel Sheet and subjected to statistical analysis using IBM Statistical Package for the Social Sciences v23. An intra-group comparison of means of microhardness and SH was performed using a paired t-test. Inter-group comparison of the parameters was done by one-way Analysis of Variance (ANOVA) and post hoc Tukey test.
RESULTS
The microhardness [Table 1] and SR [Table 2] values of the samples at baseline, demineralization, and post-remineralization time points are tabularized below. At baseline and after demineralization, there was no statistically significant difference (P > 0.05) in the microhardness and SR values of the three groups.
| Group | Interval | Mean | SD | Difference | P-value |
|---|---|---|---|---|---|
| Group A (Fluoride dentifrice) | Baseline | 326.80 | 5.05 | 165.10 | <0.001* |
| Demineralization | 161.70 | 7.92 | |||
| Demineralization | 161.70 | 7.92 | −82.70 | <0.001* | |
| Remineralization | 244.40 | 6.80 | |||
| Group B (Theobromine gel) | Baseline | 330.80 | 7.66 | 198.70 | <0.001* |
| Demineralization | 132.10 | 5.38 | |||
| Demineralization | 132.10 | 5.38 | −49.10 | <0.001* | |
| Remineralization | 181.20 | 6.49 | |||
| Group C (Nano-hydroxyapatite paste) | Baseline | 332.00 | 4.97 | 183.10 | <0.001* |
| Demineralization | 148.90 | 5.26 | |||
| Demineralization | 148.90 | 5.26 | −70.00 | <0.001* | |
| Remineralization | 218.90 | 5.53 |
Paired t-test; * indicates significant difference at P≤0.05. SD: Standard deviation
| Group | Interval | Mean | SD | Difference | P-value |
|---|---|---|---|---|---|
| Group A | Baseline | 0.61 | 0.05 | −0.81 | <0.001* |
| Demineralization | 1.42 | 0.09 | |||
| Demineralization | 1.42 | 0.09 | 0.32 | <0.001* | |
| Remineralization | 1.10 | 0.04 | |||
| Group B | Baseline | 0.65 | 0.04 | −0.61 | <0.001* |
| Demineralization | 1.26 | 0.09 | |||
| Demineralization | 1.26 | 0.09 | 0.36 | <0.001* | |
| Remineralization | 0.90 | 0.04 | |||
| Group C | Baseline | 0.60 | 0.05 | −0.56 | <0.001* |
| Demineralization | 1.16 | 0.06 | |||
| Demineralization | 1.16 | 0.06 | 0.37 | <0.001* | |
| Remineralization | 0.79 | 0.06 |
Paired t-test; * indicates significant difference at P≤0.05. SD: Standard deviation
Change in microhardness
In all three groups, the microhardness value decreased significantly (P < 0.001) after demineralization and again increased significantly (P < 0.001) after remineralization [Figure 2]. However, after remineralization, the mean microhardness values among the three groups differed significantly from each other, being most significant for fluoride dentifrice (244.4 + 6.8) and lowest for the theobromine group (181.2 + 6.49).
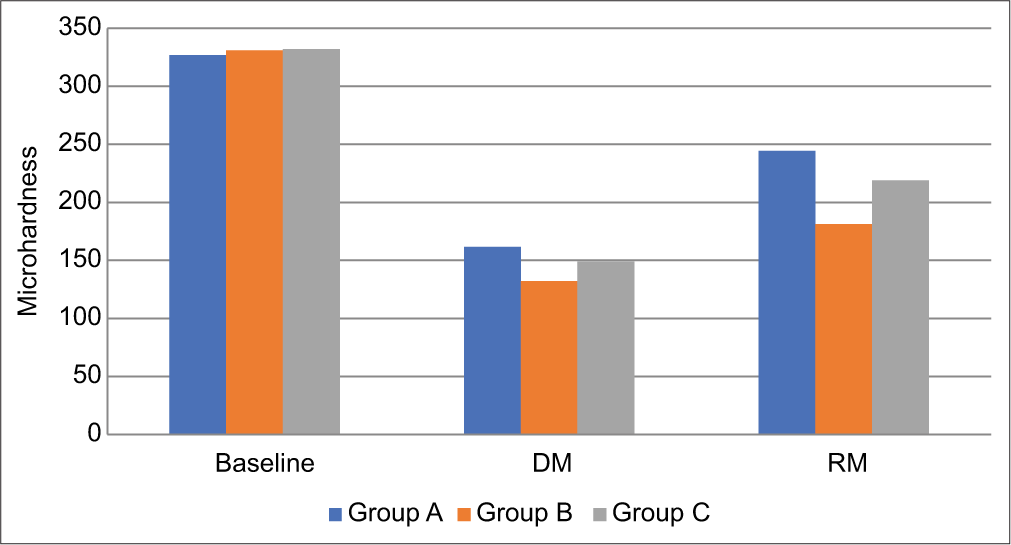
- Intergroup comparison of microhardness of three groups (DM: Demineralization, RM: Remineralization).
The highest increase in microhardness percentage was seen in Group A, followed by Group C, and was lowest in Group B. Group B showed significantly less (P < 0.05) increase in microhardness as compared to Groups A and C. Pair-wise comparison by one-way ANOVA test and post hoc Tukey test [Tables 3 and 4] revealed that after remineralization, the microhardness value of Group A was significantly higher (P < 0.05) than other two groups, and the microhardness value of Group C was significantly higher (P < 0.05) than Group B.
| Interval | Group A versus Group B | Group A versus Group C | Group B versus Group C | |
|---|---|---|---|---|
| Baseline | 0.314 | 0.149 | 0.897 | |
| DM | <0.001* | <0.001* | <0.001* | |
| RM | <0.001* | <0.001* | <0.001* | |
| Group | Mean | SD | P-value | Pairwise comparison |
| Group A | 51.40 | 7.03 | <0.001* | Gr A versus Gr B: <0.001* |
| Group B | 37.31 | 6.02 | Group A versus Gr C: 0.376 | |
| Group C | 47.23 | 7.48 | Gr B versus Gr C: 0.009* | |
One-way ANOVA test; post hoc Tukey test; * indicates significant difference at P≤0.05. DM: Demineralization, RM: Remineralization, SD: Standard deviation
| Interval | Group A | Group B | Group C | P-value | |||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | ||
| Baseline | 0.61 | 0.05 | 0.65 | 0.04 | 0.60 | 0.05 | 0.130 |
| DM | 1.42 | 0.09 | 1.26 | 0.09 | 1.16 | 0.06 | <0.001* |
| RM | 1.10 | 0.04 | 0.90 | 0.04 | 0.79 | 0.06 | <0.001* |
| Group | Mean | SD | P-value | Pairwise comparison | |||
| Group A | 22.27 | 4.60 | 0.001* | Gr A vs. Gr B: 0.023* | |||
| Group B | 28.63 | 4.88 | Group A vs. Gr C: 0.001* | ||||
| Group C | 31.44 | 5.54 | Gr B vs. Gr C: 0.423 | ||||
One-way ANOVA test; post hoc Tukey test; * indicates significant difference at P≤0.05. DM: Demineralization, RM: Remineralization, SD: Standard deviation
Change in SR
In all three groups, the SR value increased significantly (P < 0.001) after demineralization and again decreased significantly (P < 0.001) after remineralization. The highest change was seen in Group C, followed by Group B, and the lowest in Group A. Group A showed significantly less change in SR score than Group B and Group C [Table 2]. After remineralization, the SR score of Group A was significantly higher than the other two groups, and the SR score of Group B was significantly higher than Group C.
The images obtained after SEM imaging at various time points are depicted in Figure 3.
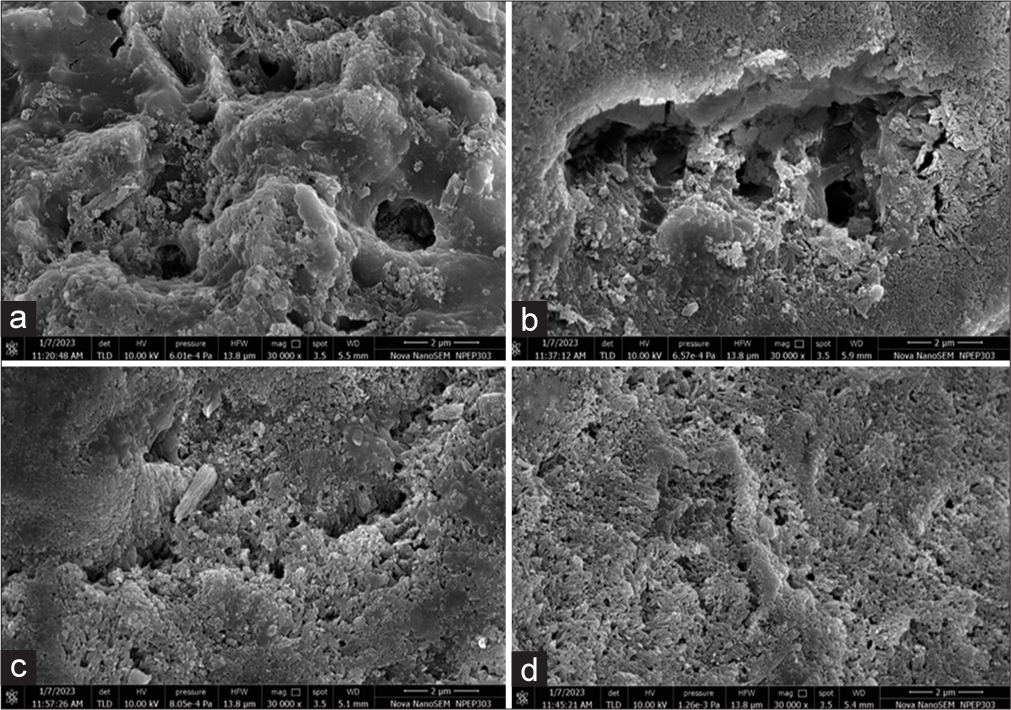
- FE-SEM analysis at ×30,000 – (a) demineralized surface, (b) remineralization with sodium fluoride, (c) theobromine gel, and (d) nano-hydroxyapatite paste. FE-SEM: Field emission scanning electron microscopy.
DISCUSSION
The present study investigated the effect of theobromine and NHA on the microhardness and SR of demineralized enamel as compared to sodium monofluorophosphate. The choice of materials precluded the potential of theobromine for remineralization despite its unpopularity and NHA represents advancements in the technology of remineralization research. It was, therefore, necessary to simulate a clinical condition of demineralized enamel in the oral environment for which the precise above-mentioned methodology was employed. The pH cycling model used in the present study was adopted from the regimen described by Gocmen et al. based on the model introduced by Dunipace et al. for the evaluation of remineralization properties of enamel.[12,13]
The microhardness was measured using Vicker’s microhardness test. The selection of this particular test was due to its versatility, accuracy, and reliability while requiring some effort for surface preparation of the sample. A similar selection was done in earlier studies that evaluated enamel remineralization of tooth samples.[13,14]
The SR was measured using a stylus profilometer. It is a device used for analyzing topographical characteristics of the surface of materials but requires thorough contact and causes surface destruction.[15] Since the present in vitro study was conducted on extracted teeth, this particular technique was suitable for measuring the SR. Likewise, earlier researchers who used extracted teeth to study the enamel SR have successfully employed this technique.[16,17]
At baseline and after demineralization, there was no difference in microhardness between the three groups, indicating that the groups were identical at the beginning of the study and after demineralization. In all three groups, the microhardness value decreased significantly, implying that the demineralization process was successfully performed. A significant increase in the microhardness after remineralization in all three groups indicated that all three agents are effectively achieved remineralization of the lost enamel structure. The highest increase in microhardness percentage was seen in the teeth treated with sodium monofluorophosphate followed by NHA and theobromine. In fact, theobromine achieved significantly less percentage increase in microhardness than the other two agents. Our findings corroborate those reported by Najibfard et al., who stated that NHA and fluoride dentifrice achieved significant remineralization.[18] A similar recent study reported that brushing with sodium monofluorophosphate provided the highest increase in microhardness among other dentifrices containing theobromine or caffeine.[19] Premnath et al. found the remineralization potential of fluoride dentifrice to be higher than theobromine, similar to the the present research findings.[20] The significant decrease in roughness achieved by the NHA group observed in Mielczarek et al.’s study is similar to our findings.[21]
On the contrary, earlier studies compared theobromine to standard sodium fluoride dentifrices. The results showed that there was a greater increase in SMH value of enamel achieved by theobromine compared to the latter. In 2018 Suryana et al. also found that the enamel hardness resulting from the use of theobromine was significantly higher than that resulting from the use of NHA.[22] Taneja et al., on the other hand, did not find any significant difference in the remineralizing capacity of the three agents.[7]
At baseline under SEM, the surface appeared to be almost smooth with very sparsely located pores, and after the demineralization, it appeared as a homogenous wide-scale pattern of honeycomb/micropores. Following remineralization in the case of the sodium monofluorophosphate, not all the pores were sedimented, especially when compared to theobromine and NHA, wherein the surface appeared smooth with filled pores. The better filling of the demineralized voids by NHA can be attributed to the nano-sized particles that clinically manifest as improved microhardness and a smoother surface.[23] A similar correlation was found by Amaechi et al. wherein the authors reported that more of the tubules were occluded with the theobromine when compared to the sodium monofluorophosphate when observed under SEM.[24]
While the present study offers plausible results, certain limitations of its design cannot be overlooked. The oral cavity harbors numerous biological and chemical entities that cannot be entirely replicated in vitro.[25,26] Since the teeth are bathed in varying quantities and combinations of salivary components, the pH cycling model used in the present study cannot accurately simulate the saliva and plaque fluids existing in the natural state.[26,27] The demineralization process induced in the in vitro design is much faster than that which occurs in the oral cavity. Long-term in vivo studies with adequate follow-up can help overcome these limitations.
CONCLUSION
The present study demonstrates that sodium monofluorophosphate, theobromine, and NHA are all effective remineralizing agents for demineralized enamel. The results indicated that while all three agents significantly increased the microhardness of demineralized enamel, sodium monofluorophosphate achieved the greatest increase in microhardness, followed by NHA, with theobromine showing the slightest increase. Specifically, the mean microhardness values post-remineralization were highest for sodium monofluorophosphate, intermediate for NHA, and lowest for theobromine.
All three agents significantly decreased the SR after remineralization. NHA showed the most significant improvement in SR, followed by theobromine, with sodium monofluorophosphate showing the least improvement. The SR scores post-remineralization were highest for sodium monofluorophosphate, intermediate for theobromine, and lowest for NHA. The SEM images further supported these findings, showing that NHA and theobromine more effectively filled the demineralized pores compared to sodium monofluorophosphate, resulting in smoother surfaces.
These results suggest that while sodium monofluorophosphate is highly effective in enhancing microhardness, NHA excels in improving microhardness and reducing SR. Therefore, NHA may offer a superior balance of benefits in remineralizing demineralized enamel. Future in vivo studies with long-term follow-up are recommended to confirm these findings and better simulate the natural oral environment.
Ethical approval
The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Institutional Ethical Review Board (BVDU/DCH/620- 1/2020-2021).
Declaration of patient consent
Patient’s consent was not required as there are no patients in this study.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship: Nil.
References
- Dental Caries. In: Shafer’s Textbook of Oral Pathology (9th ed). India: Elsevier RELX India Pvt Ltd; 2020. p. :369-403. [An Adaptation of A Textbook of Oral Pathology 1983, 4th ed, Elsevier Inc.]
- [Google Scholar]
- Demineralization and Remineralization of Teeth New York: EduBubs Publishing House; 2020.
- [Google Scholar]
- The Effects of Calcium, Magnesium, Phosphorus, Fluoride, and Lead on Bone Tissue. Biomolecules. 2021;11:506.
- [CrossRef] [PubMed] [Google Scholar]
- Mechanisms of Fluoride Toxicity: From Enzymes to Underlying Integrative Networks. Appl Sci. 2020;10:7100.
- [CrossRef] [Google Scholar]
- A Systematic Review and Meta-Analysis of the Relationship between the Severity of Dental Fluorosis and Fluoride Biomarkers in Endemic Areas. Biol Trace Elem Res. 2023;201:1051-62.
- [CrossRef] [PubMed] [Google Scholar]
- Remineralization Potential of Theobromine on Artificial Carious Lesions. J Int Soc Prev Community Dent. 2019;9:576-83.
- [CrossRef] [PubMed] [Google Scholar]
- Nanohydroxyapatite in Dentistry: A Comprehensive Review. Saudi Dent J. 2023;35:741-52.
- [CrossRef] [PubMed] [Google Scholar]
- Nano-Hydroxyapatite (nHAp) in the Remineralization of Early Dental Caries: A Scoping Review. Int J Environ Res Public Health. 2022;19:5629.
- [CrossRef] [PubMed] [Google Scholar]
- Enamel Hardness Differences after Topical Application of Theobromine Gel and Casein Phosphopeptide-amorphous Calcium Phosphate. Conserv Dent J. 2020;10:5-8.
- [CrossRef] [Google Scholar]
- pH-Cycling Models for In Vitro Evaluation of the Efficacy of Fluoridated Dentifrices for Caries Control: Strengths and Limitations. J Appl Oral Sci. 2010;18:316-34.
- [CrossRef] [PubMed] [Google Scholar]
- Effectiveness of Some Herbals on Initial Enamel Caries Lesion. Asian Pac J Trop Biomed. 2016;6:846-50.
- [CrossRef] [Google Scholar]
- An In Vitro Model for Studying the Efficacy of Fluoride Dentifrices in Preventing Root Caries. Caries Res. 1994;28:315-21.
- [CrossRef] [PubMed] [Google Scholar]
- Qualitative and Quantitative Comparison of the Remineralisation Potential of Three Suitable Materials-An In Vitro SMH and SEM Study. J Clin Diagn Res. 2019;13:ZC01-4.
- [CrossRef] [Google Scholar]
- Assessment of Surface Profile Data Acquired by a Stylus Profilometer. Meas Sci Technol. 2012;23:105601.
- [CrossRef] [Google Scholar]
- Comparison of Remineralizing Effect of Organic and Inorganic Fluoride by Evaluation of Microhardness and Quantitative Analysis of Calcium and Phosphorus Ratio on Enamel Surface: An In-vitro Study. Int J Dent Mater. 2020;2:75-81.
- [CrossRef] [Google Scholar]
- Changes in Surface Roughness of Bleached Enamel by Using Different Remineralizing Agents. Tanta Dent J. 2016;13:179.
- [CrossRef] [Google Scholar]
- Remineralization of Early Caries by a Nano-hydroxyapatite Dentifrice. J Clin Dent. 2011;22:139-43.
- [Google Scholar]
- The Effect of Toothpastes Containing Natural Ingredients Such As Theobromine and Caffeine on Enamel Microhardness: An In Vitro Study. Evid Based Complement Alternat Med. 2021;2021:3304543.
- [CrossRef] [PubMed] [Google Scholar]
- Effectiveness of Theobromine on Enamel Remineralization: A Comparative In-vitro Study. Cureus. 2019;11:e5686.
- [CrossRef] [PubMed] [Google Scholar]
- The Effect of Nano-hydroxyapatite Toothpaste on Enamel Surface Remineralization. An In Vitro Study. Am J Dent. 2014;27:287-90.
- [Google Scholar]
- The Effects of Toothpastes Containing Theobromine and Hydroxyapatite On Enamel Microhardness after Immersion in Carbonated Drink. J Phys Conf Ser. 2018;1073:032010.
- [CrossRef] [Google Scholar]
- Nanohydroxyapatite and Its Applications in Preventive, Restorative and Regenerative Dentistry: A Review of Literature. Ann Stomatol (Roma). 2014;5:108-14.
- [CrossRef] [Google Scholar]
- Remineralization of Artificial Enamel Lesions by Theobromine. Caries Res. 2013;47:399-405.
- [CrossRef] [PubMed] [Google Scholar]
- Oral Microbiome: Unveiling the Fundamentals. J Oral Maxillofac Pathol. 2019;23:122-8.
- [CrossRef] [PubMed] [Google Scholar]
- Saliva as Research Material: Biochemical, Physicochemical, and Practical Aspects. Arch Oral Biol. 2007;52:1114-35.
- [CrossRef] [PubMed] [Google Scholar]
- Correction: Chemical Analysis in Saliva and the Search for Salivary Biomarkers-A Tutorial Review. Analyst. 2018;143:777-83.
- [CrossRef] [PubMed] [Google Scholar]







