Translate this page into:
Cholesterol Crystals in Odontogenic Cysts: An In-Depth Narrative of Pathogenesis

*Corresponding author: Sanpreet Singh Sachdev, Department of Oral Pathology and Microbiology, Government Dental College and Hospital, Mumbai, Maharashtra, India. sunpreetss@yahoo.in
-
Received: ,
Accepted: ,
How to cite this article: Sachdev SS, Singh P, Chettiankandy YJ, Trimukhe A, Sachdev JB, Richhawal A. Cholesterol Crystals in Odontogenic Cysts: An in-Depth Narrative of Pathogenesis. Glob J Med Pharm Biomed Update 2023;18:26.
Abstract
Cholesterol crystals (CCs) are a relatively common finding in infected odontogenic cysts occurring in about 60% of the cases. Histopathologically, these appear as longitudinal cleft-like spaces or may appear as a collection in cholesterol granulomata. The occurrence of CCs in odontogenic cysts is closely associated with the pathophysiology of the lesions. Despite being fairly common, not much is known about the sources of cholesterol, the mechanism of formation of these clefts, their pathogenesis, and their clinical implications. The present article focuses on describing CCs with respect to their contents, pathogenesis, and clinical implications. Our review describes the mechanism of the formation of CCs in a detailed manner along with a pictorial depiction of the cascade of events involved in the process. The formation of cholesterol granulomata is a cumulative effect of the breakdown of erythrocytes and leukocytes, intramural hemorrhage, decreased blood supply and lymphatic drainage, hypoxic conditions, increased vascular permeability, accumulation and rupture of lipid-laden macrophages, and foreign body reaction. It would be of great interest to pathologists to have an in-depth understanding of the pathogenesis, nature, and implications of CCs, which are very common yet enigmatic entities.
Keywords
Odontogenic lesions
Cholesterol granuloma
Pathophysiology
Histopathology
Lipids
INTRODUCTION
Cholesterol is a steroid lipid that forms one of the primary constituents of the cell membranes of animal cells. Degeneration of lipids present in the tissues leads to precipitation products in the form of cholesterol crystals (CCs), noted commonly in inflammatory lesions.[1] In oral pathology, CCs are commonly associated with inflammatory cysts, infected developmental odontogenic cysts, and periapical granulomas.
The incidence of intramural CCs has been reported to vary from 18% to 44% of odontogenic cysts by various researchers in different samples.[1,2] The frequency could be higher if entire cyst linings were examined instead of only random sections that are observed in routine practice. Browne found them to be suspended in the cystic fluids of 60% of odontogenic cysts, including 78% of dentigerous cysts and 67% of odontogenic keratocysts.[3]
When present in cystic lesions, these CCs impact a shimmering gold color to the luminal fluid under sunlight [Figure 1a], and when the fluid is observed directly under the microscope, the crystals appear rectangular in shape with notched corners [Figure 1b]. These crystals characteristically emit blue and yellow light when observed under a polarized microscope.
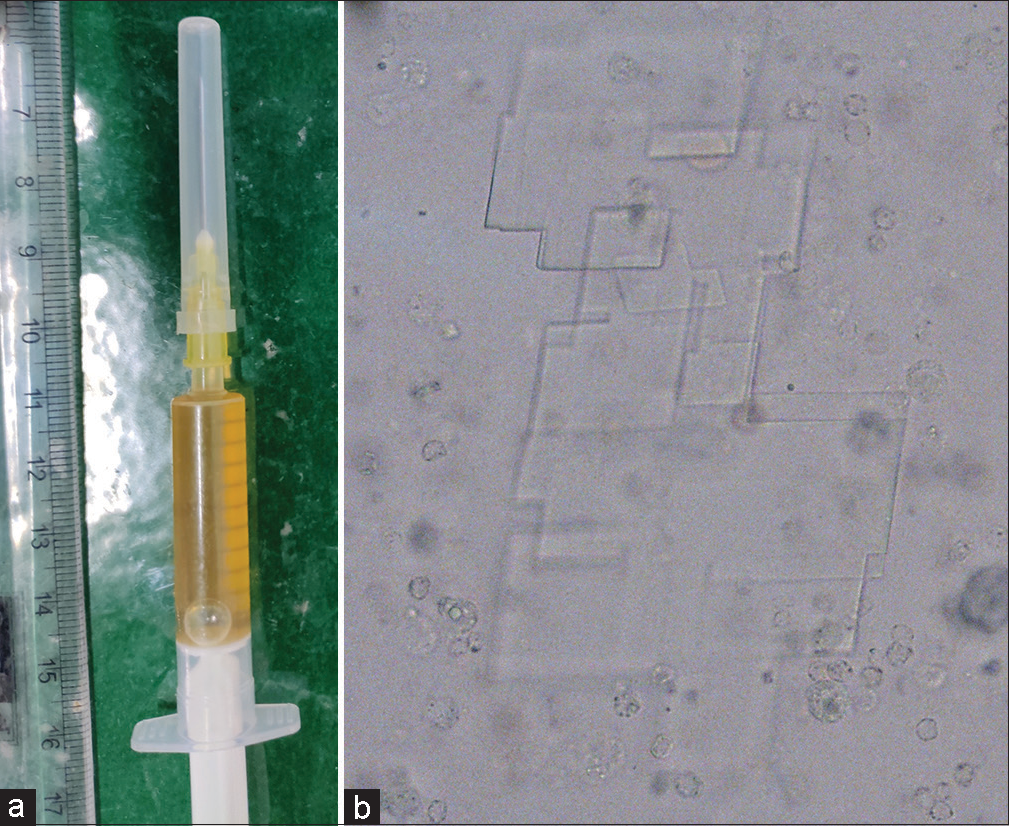
- (a) Shimmering gold-colored aspirate and (b) rectangular cholesterol crystals with a notched corner when observed under a microscope (original magnification ×400).
Histopathologically, the CCs appear as longitudinal cleft-like spaces due to the dissolution of cholesterol in the fat solvents used during tissue processing [Figure 2]. Arwill and Heyden (1973) demonstrated that the crystals accumulate in congested capillaries in the portions of a cystic wall having inflammation.[4] This would explain their appearance as needle-like or cleft-like structures being enveloped by endothelial cells. These cholesterol clefts (CCls) commonly appear congregated in certain areas of the cyst wall or connective tissue stroma. [Figure 2] Frequently, but not always, foreign body giant cells [Figure 3], foamy histiocytes, areas of hemorrhage, and macrophages rich in hemosiderin are observed adjacent to the CCls.[5]
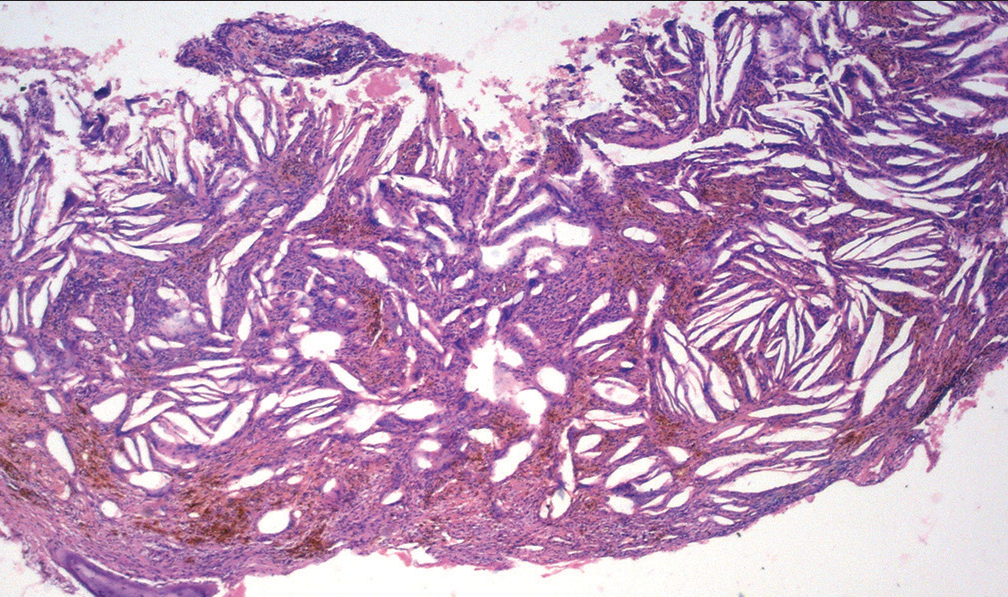
- Histopathological picture of a cholesterol granuloma (H and E, original magnification ×100).
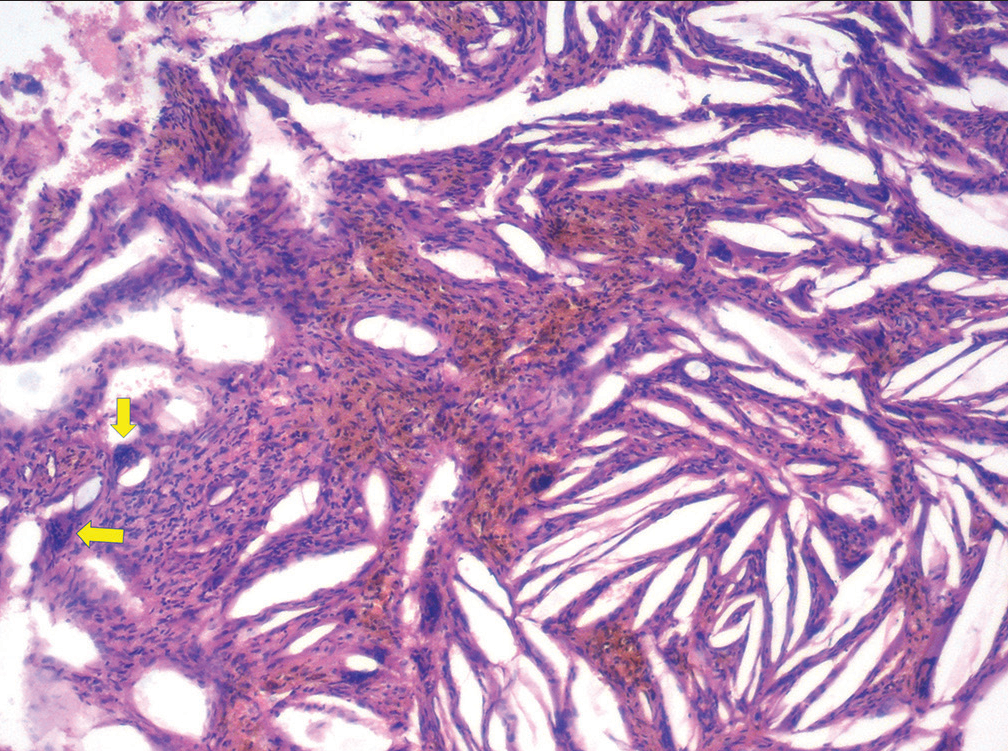
- Higher magnification showing multinucleated giant cells (arrows) and hemorrhagic elements associated with cholesterol clefts (H and E, original magnification ×400).
Apart from occasional scattered clefts, a collection of CCls may occur in an area of the cystic wall associated with granulation tissue, which is commonly referred to as “cholesterol granulomata.”[6] It appears as a “mural nodule” when the overlying lining epithelium ulcerates and the cholesterol protrudes into the cystic lumen. Some degree of inflammatory changes is often associated with the concentrated deposits of the cholesterol granulomata. However, no correlation has yet been found between the extent of intramural inflammation and the deposition of cholesterol.
Despite being fairly common, not much is known about the pathogenesis of CCls, possibly because not much research work has been directed toward them. The little amount of work available on the CCls in odontogenic cysts was conducted by the end of the 20th century. The present article focuses on describing CCs with respect to their contents, pathogenesis, and clinical implications.
PATHOGENESIS
The concept that cholesterol is derived from the degeneration of erythrocytes and their digestion by macrophages is widely accepted.[2] RBCs contain cholesterol in their plasmalemma which has been found to be of the order of 5.2 × 1013 g/cell.[7] Invariably,hemosiderin was found to be present in the walls of all odontogenic cysts even in those which did not show the presence of intramural cholesterol. Much of the staining for hemosiderin has been found to be more concentrated in and around the macrophages and particularly conspicuous within and around CCls.[8]
In general, macrophages and giant cells are capable of breaking down CCs as long as they can be completely engulfed. The macrophages possess mechanisms for the uptake of lipoproteins and the ingestion and digestion of cholesterol esters.[6] Perlecan is a basement membrane-specific heparan sulfate proteoglycan that entraps the low-density lipoproteins.[9] The accumulated complex is then oxidized in the extracellular space, scavenged by macrophages, and deposited inside the cells, converting them into lipid-laden foamy cells.[6,9]
The foamy cells transform the hydrophobic CCs into a soluble form by incorporating them into lipoprotein vehicles.[1] The esterified lipoprotein-bound cholesterol has an affinity for the surface receptors of macrophages which facilitate its endocytosis. The cholesterol esters are then delivered to the lysosomes and hydrolyzed by acid lipase to form cholesterol and fatty acids. This represents the “first cellular compartment” stage of the metabolism of cholesterol in the macrophages.[10] This would account for the absence of cholesterol in cases (about 19%), in which hemosiderin is still detected.[2] The cholesterol ultimately gets stored as cholesterol oleate in the lipid droplets. Re-esterification of the cholesterol by microsomal enzymes occurs in the “second cellular compartment” stage [Figure 4].
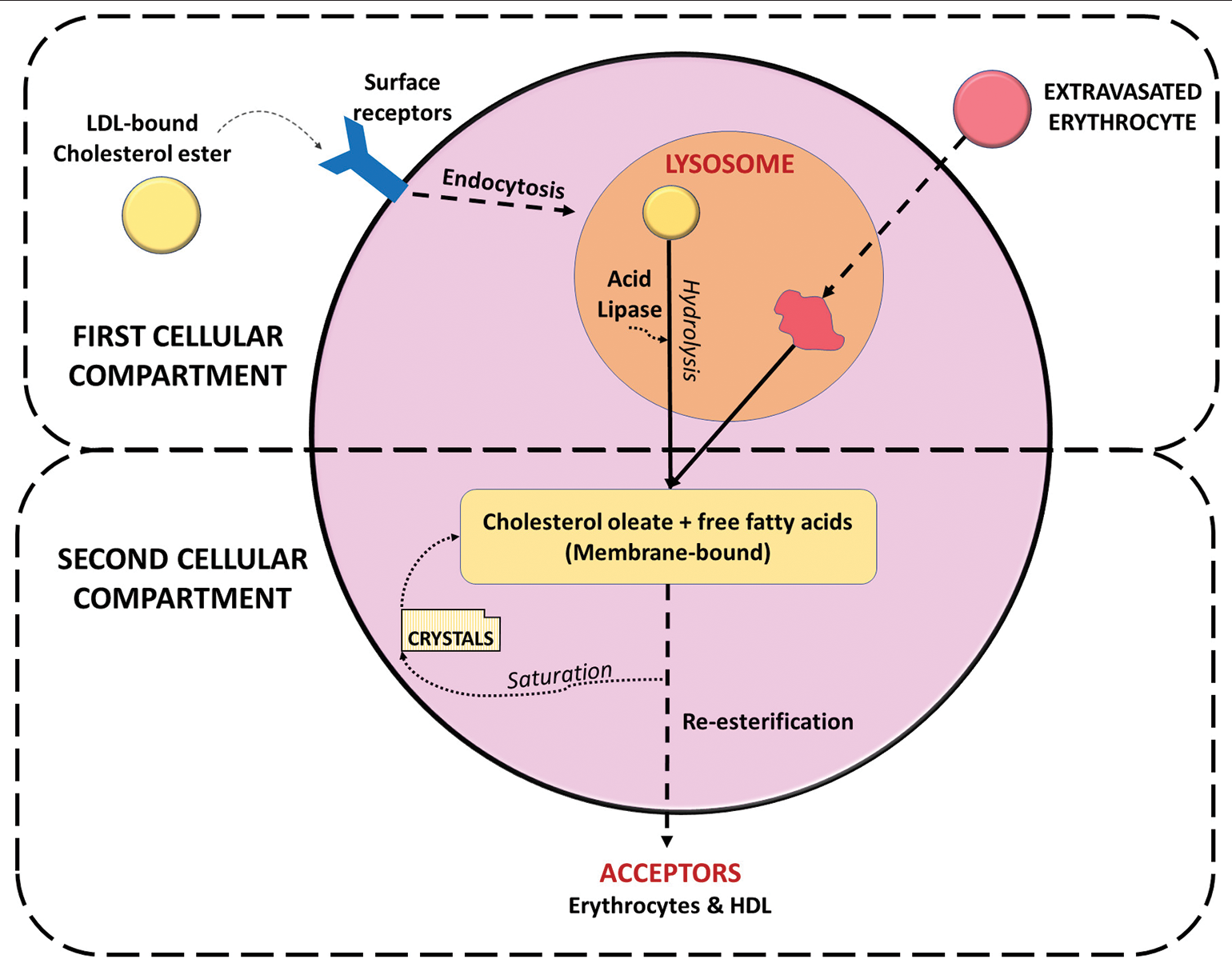
- Alteration and offloading of cholesterol in foamy cells under physiologic conditions.
Evidence of overloading of cholesterol with crystallization has been detected in the foamy cells adjacent to the necrotic areas due to hypoxic conditions. The processes in the second cellular compartment allow the foamy cells to offload their cholesterol under conditions of high loading in the form of micelles. The high-density lipoproteins and erythrocytes have receptors present on their surfaces that serve as acceptors for the offloaded cholesterol.[11] Therefore, under natural conditions, the offloading process is dependent on a constant blood supply that regularly delivers and removes these acceptors.
The offloading in itself gets impaired when the provision of acceptors from the circulating blood is reduced. Such a situation may arise when there is stagnation of vascular supply to the cyst wall due to a large area of hemorrhage.[12] The extravasation of blood elements would, further, add to the hydrostatic pressure, causing impedance to the passage of blood through the vessels adjacent to the cystic capsule.
Extravasated erythrocytes have been identified as even more potent acceptors of cholesterol that are able to stimulate its excretion from the foamy cells.[13] Therefore, the presence of abundant erythrocytes would create a “Trojan Horse” situation for the foamy histiocytes causing them to allow endocytosis of more amount of cholesterol than under normal conditions. The stagnant or extravasated erythrocytes would also accept more cholesterol in the lipid-containing milieu, than the circulating cells.
Even so, intramural hemorrhages are not always associated with the formation of CCls. This is because the normal tissue mechanisms for handling hemorrhages are sufficient to prevent excessive cholesterol deposition. This mechanism, however, gets impaired in cystic lesions due to their peculiar anatomy and pathophysiology. In combination with the poor blood supply and lymphatic drainage of the cystic walls, the hemorrhage causes hypoxia to more pronounced.[2,10,12]
An additional effect of the hypoxic condition would be impairment of the functional efficacy of the foamy cells. A cumulative effect of all these factors beyond a certain threshold would render the foamy cells unable to digest the cholesterol completely. Therefore, the foamy cells themselves become responsible for outbursts of cholesterol and their accumulation in the tissue spaces. It is also not surprising that foamy macrophages have not been detected in all the cysts that contain cholesterol, for their phase of activity, which may have passed before the time of extension and are most likely to have done their task efficiently. Even so, previous attempts at the metabolism of the cholesterol esters by the foam cells were demonstrated by acid esterase activity around some of the lipid droplets.[10]
SOURCES OF CHOLESTEROL
Although large amounts of lipids may be found in the foamy cells, it is highly unlikely that the entirety of cholesterol originates exclusively from the erythrocytes. Many foamy cells or clefts are not always associated with many red cells or much hemosiderin. Electron Probe Elemental Analyses suggested that lysosomal acid phosphatase may occur in association with residues from an intracellular breakdown of red cells; a testimony that such foam cells at one stage ingested red cells.[10] The amount of hemosiderin present in cyst walls has been found to vary considerably, unrelated to the amount of cholesterol present.
A potential source of cholesterol as described by Shear (1963) and Skaug (1977), which closely resembles the mechanism of formation of atherosclerosis, is the circulating plasma lipids.[14,15] It has been demonstrated that CCs increase the permeability of the endothelium in a dose- and time-dependent manner.[16] CCs lead to the production of hydrogen peroxide which, in turn, leads to inactivation of Src homology region 2 containing protein tyrosine phosphatase. This induces tyrosine phosphorylation of vascular E-cadherin and b-catenin. As a consequence, the adherent junctions present between the endothelial cells get disrupted. The resultant increased vascular permeability allows high-density lipids to leak out from the bloodstream into the extracellular spaces. This provides an increased number of acceptors for the offloading of foamy cells and coupled with poor lymphatic drainage of the cyst wall, may contribute to the intramural accumulation of CCs.
Most of the studies investigating the contents of CCs were carried out in the late 1900s.[2,17,18] The findings by various authors and their implications in determining the sources of cholesterol are summarized in [Table 1].
| S. No. | Author | Components found | Interpretation for the source of cholesterol |
|---|---|---|---|
| Shear (1963)[14] Skaug (1977)[15] |
β-lipoproteins | From circulating plasma lipids | |
| Browne (1971)[17] | Hemosiderin and cholesterol esters in adjacent giant cells | Breakdown of red blood cells | |
| Trott et al. (1973)[18] | Phospholipids | Disintegration of lymphocytes, plasma cells, and macrophages | |
| Arwill and Heyden (1973)[4] | Intercellular adhesion molecule-1 and endothelial leucocyte adhesion molecule-1 | Crystals may form in congested capillaries in inflamed areas as they appear to be enveloped by endothelial cells. | |
| Yamazaki et al. (2004)[9] | basement membrane-type heparin, perlecan, and low-density lipoprotein | From degenerated basement membrane and lining epithelial cells |
CONTENTS OF THE CRYSTALS
Histochemistry
When the sections containing CCs are pretreated with digitonin, cholesterol digitonide is formed, indicative of the presence of free cholesterol. Further, treatment with acetone washes out other lipids except for free cholesterol which can be demonstrated by its positive staining by perchloric acid-naphthoquinone.[10]
Further, support for the free state of cholesterol is provided by positive uptake of Sudan black following the liquefaction of the crystals by bromination reaction.[19] Cholesterol esters were found in the foamy histiocytes by observing under a polarizing microscope after staining with Oil Red O. The globular birefringent material detected is attributable to the presence of crystals of cholesterol ester and triglycerides.[10]
Ultrastructural findings
Electron microscopy has revealed that not all CCls are associated with giant cells or macrophages and occasional clefts lie in tissue spaces packed with foamy macrophages. The giant cells associated with the CCs are rich in lysosomes, which stain positively by acid phosphatase. They contain numerous lysosome-like structures, mitochondria, and variable amounts of endoplasmic reticulum but intracellular lipid was not found when observed for their ultrastructure. Other inflammatory cells and histiocytes also exhibit acid phosphate content but the reaction is most intense in the multinucleated giant cells. A more scattered distribution in the foam cells is most likely due to the large lipid droplets present between the protoplasm.[10]
The thin crystals of cholesterol occupy spaces with remarkably flat sides and somewhat rounded ends. In most instances, the large CCls are incompletely associated with a foreign body giant cell, leaving one end free. A narrow strip of debris often exists between the outer length of the space left by the crystal cleft and the associated giant cell.[10] The tip of crystals is associated with dense arrays of collagen fibers if not occupied by a giant cell. Red cells and their remains were frequently found adjacent to the parts of the cleft. Droplets of lipid are at times seen lying free within such tissue spaces and so are likely to have arisen from the breakdown of a foam cell.
CCls have also been observed in the midst of necrotic debris that contains only a few scattered foamy cells and an absence of giant cells.[20] Many of the foam cells in these situations also have been found to contain intracellular crystalline clefts that vary in number and size. Star-like crystalline structures are also seen within membrane-bound electron-dense surrounds and it is tempting to consider that these crystals are in the process of being digested.[21] A crystalline cleft may appear to be only partially contained within a foamy macrophage but it is, of course, impossible to say whether this represents engulfment or extrusion.
While the smaller CCs are easily ingested by the foamy cells, larger crystals resist internalization. As a result, the macrophages are unable to endocytose the crystals circumfuse to form multinucleated giant cells. In this manner, the larger CCs behave as a stimulus for eliciting a foreign body response.[6] This explains why not all cases with intramural CCls are always associated with a giant cell reaction.
The histological, histochemical, and ultrastructural findings do not support the general belief that the deposition of cholesterol is merely an accumulation from extravasated red blood cells but it appears to be the consequence of the complex set of events. The entire cascade of events is shown in [Figure 5].
It would be of interest to determine whether foamy macrophages have a role in other conditions showing frequent hemorrhages such as giant cell granuloma or aneurysmal bone cyst. Although there occurs a considerable deposition of hemosiderin in these conditions, accumulation of CCs does not occur. Quite possibly, this would be due to foamy cells being able to carry out their function effectively and the presence of sufficient blood supply and lymphatic drainage to remove any excess lipids.
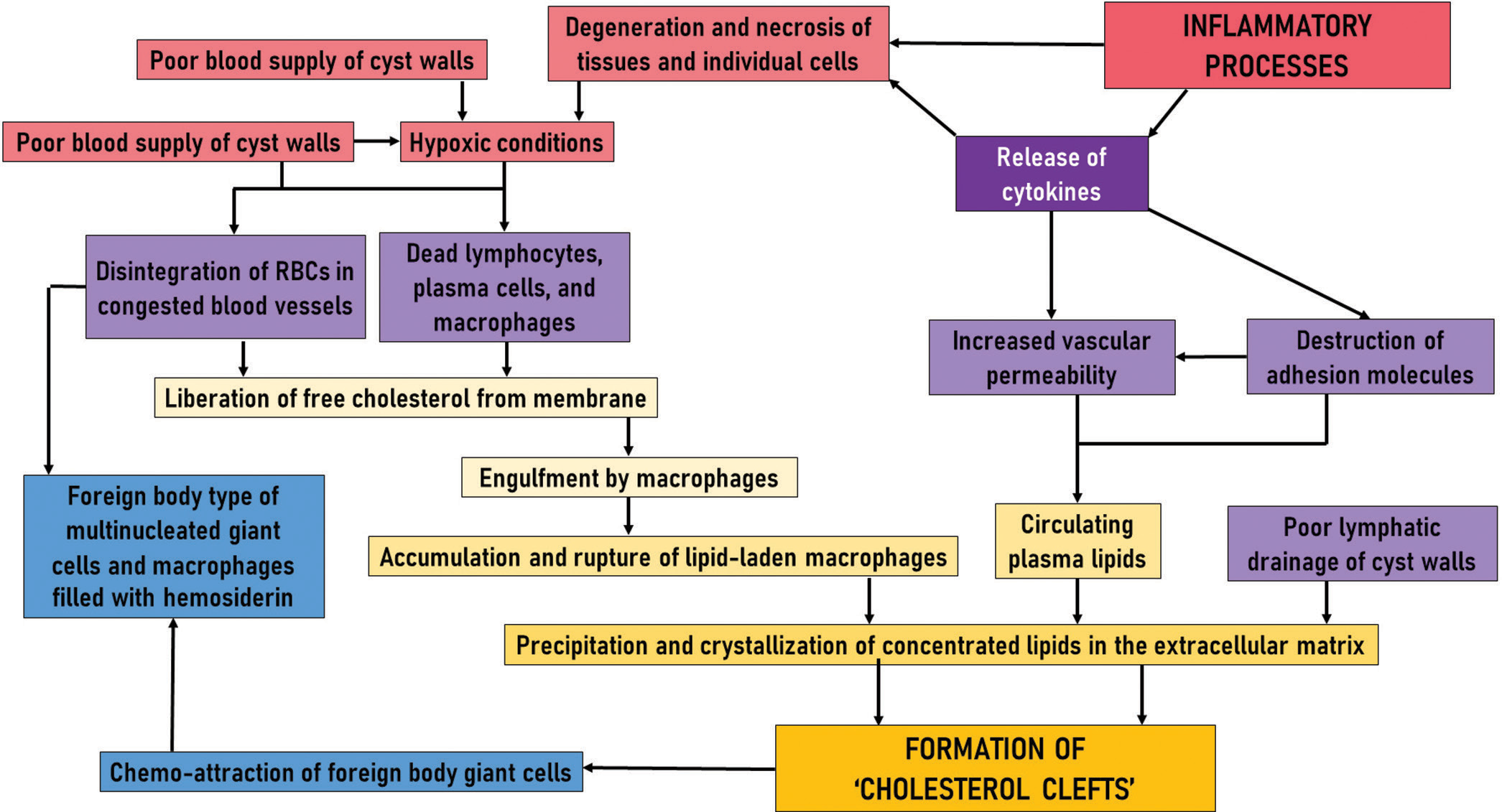
- Cascade of events involved in the formation of cholesterol clefts in odontogenic cysts.
Clinical implications
CCs deposited in the cystic capsules are capable of exciting a foreign body response along with the release of cytokines including interleukin (IL)-1a, IL-1b, and IL-6 in the epithelial lining and vascular endothelial cells, and tumor necrosis factor and IL-8 in macrophages.[2] As a consequence, a number of clinical implications have been noted in relation to the functions of the released cytokines:
It has been reported that odontogenic cysts having a foreign body reaction to CCs in their capsule had higher chances of becoming expansile and involving the maxillary sinus[22]
The formation of cholesterol granulomata has been identified as a driving factor for cyst expansion[9]
Accumulation of endogenous CCs that irritate periapical tissues has been identified as one of the six most important biological factors, leading to an asymptomatic periapical radiolucency persisting after a root canal treatment[1]
The macrophages and giant cells surrounding the CCs are unable to degrade them entirely. The surviving crystals elicit an inflammatory response and cause release mediators for bone resorption such as IL-1a[23]
Long-term follow-up of cases of apical periodontitis has shown that the CCs can sustain the lesion indefinitely thereby adversely affecting the post-treatment healing.[24] Even retreatment of such teeth by a non-surgical root canal would be unable to remove the CCs from the periapical tissues as they virtually exist outside the root canal system.[25]
CONCLUSION
The formation of cholesterol granulomata is a cumulative effect of the breakdown of erythrocytes and leukocytes, intramural hemorrhage, decreased blood supply and lymphatic drainage, hypoxic conditions, increased vascular permeability, accumulation, and rupture of lipid-laden macrophages, and foreign body reaction. It would be of great interest to pathologists to have an in-depth understanding of the pathogenesis, nature, and implications of CCs, which are very common yet enigmatic entities.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The author(s) confirms that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using the AI.
Financial support and sponsorship
Nil.
References
- On the Causes of Persistent Apical Periodontitis: A Review. Int Endod J. 2006;39:249-81.
- [CrossRef] [PubMed] [Google Scholar]
- Cysts of the Oral and Maxillofacial Regions. United States: John Wiley and Sons; 2008.
- [CrossRef] [Google Scholar]
- Some Observations on the Fluids of Odontogenic Cysts. J Oral Pathol. 1976;5:74-87.
- [CrossRef] [PubMed] [Google Scholar]
- Histochemical Studies on Cholesterol Formation in Odontogenic Cysts and Granulomas. Scand J Dent Res. 1973;81:406-10.
- [CrossRef] [PubMed] [Google Scholar]
- Cholesterol Granuloma of the Jaws: Report of Two Cases. J Pak Med Assoc. 2014;64:86-8.
- [Google Scholar]
- Cholesterol Granuloma in Odontogenic Cyst: An Enigmatic Lesion. Case Rep Dent. 2016;2016:6105142.
- [CrossRef] [PubMed] [Google Scholar]
- The Molecular Structure of Human Red Blood Cell Membranes from Highly Oriented, Solid Supported Multi-lamellar Membranes. Sci Rep. 2017;7:39661.
- [CrossRef] [PubMed] [Google Scholar]
- Cholesterol Granuloma in the Wall of a Mandibular Dentigerous Cyst: A Rare Case Report. J Clin Exp Dent. 2010;2:e88-90.
- [CrossRef] [Google Scholar]
- Basement Membrane-type Heparan Sulfate Proteoglycan (Perlecan) and Low-density Lipoprotein (LDL) are Co-localized in Granulation Tissues: A Possible Pathogenesis of Cholesterol Granulomas in Jaw Cysts. J Oral Pathol Med. 2004;33:177-84.
- [CrossRef] [PubMed] [Google Scholar]
- Investigative Pathology of Odontogenic Cysts. United States: CRC Press; 2019.
- [CrossRef] [Google Scholar]
- HDL and Lipid Metabolism In: HDL Metabolism and Diseases. Singapore: Springer; 2022. p. :49-61.
- [CrossRef] [PubMed] [Google Scholar]
- Lipids in the Walls and Contents of Jaw Cysts. Scand J Dent Res. 1976;84:409-12.
- [CrossRef] [PubMed] [Google Scholar]
- Variability of Cholesterol Accessibility in Human Red Blood Cells Measured using a Bacterial Cholesterol-binding Toxin. Elife. 2017;6:e23355.
- [CrossRef] [PubMed] [Google Scholar]
- Cholesterol in Dental Cysts. Oral Surg Oral Med Oral Pathol. 1963;16:1465-73.
- [CrossRef] [PubMed] [Google Scholar]
- Soluble Proteins in Fluid from Non-keratinizing Jaw Cysts in Man. Int J Oral Surg. 1977;6:107-21.
- [CrossRef] [PubMed] [Google Scholar]
- Cholesterol Crystals Increase Vascular Permeability by Inactivating SHP2 and Disrupting Adherens Junctions. Free Radic Biol Med. 2018;123:72-84.
- [CrossRef] [PubMed] [Google Scholar]
- The Origin of Cholesterol in Odontogenic Cysts in Man. Arch Oral Biol. 1971;16:107-13.
- [CrossRef] [PubMed] [Google Scholar]
- Factors Related to Cholesterol Formation in Cysts and Granulomas. J Can Dent Assoc (Tor). 1973;39:550-5.
- [Google Scholar]
- Cholesterol Crystallization within Hepatocyte Lipid Droplets and its Role in Murine NASH. J Lipid Res. 2017;58:1067-79.
- [CrossRef] [PubMed] [Google Scholar]
- Pathways of Inflammatory Cellular Exudate through Radicular Cyst Epithelium: A Light and Scanning Electron Microscope Study. J Oral Pathol Med. 1979;8:369-78.
- [CrossRef] [PubMed] [Google Scholar]
- Scanning Electron Microscopic Observations on the Inner Surface of Jaw Cysts. Int J Oral Surg. 1985;14:526-32.
- [CrossRef] [PubMed] [Google Scholar]
- Cholesterol Granuloma of the Maxillary Sinus. Braz Dent J. 2008;19:171-4.
- [CrossRef] [PubMed] [Google Scholar]
- Bone-resorbing Activity from Cholesterol-exposed Macrophages due to Enhanced Expression of Interleukin-l? J Dent Res. 2002;81:11-6.
- [CrossRef] [PubMed] [Google Scholar]
- Radicular Cyst Affecting a Root-filled Human Tooth: A Long-term Post-treatment Follow-up. Int Endod J. 1993;26:225-33.
- [CrossRef] [PubMed] [Google Scholar]
- Cholesterol as an Aetiological Agent in Endodontic Failures--a Review. Aust Endod J. 1999;25:19-26.
- [CrossRef] [PubMed] [Google Scholar]







