Translate this page into:
Antifungal Effects of Thymol-Loaded Chitosan Nanocomposite Alone and in Combined with Nystatin Against Candida Albicans, a Major Cause of Oral Candidiasis

*Corresponding author: Asghar Sepahvand, Razi Herbal Medicines Research Center, Lorestan University of Medical Sciences, Khorramabad, Iran. fungimed44@yahoo.com
-
Received: ,
Accepted: ,
How to cite this article: Azadbakht K, Hadipour S, Rashidipour M, Sepahvand A. Antifungal effects of thymol-loaded chitosan nanocomposite alone and combined with nystatin against Candida albicans, a major cause of oral candidiasis. Glob J Med Pharm Biomed Update 2022;17:7.
Abstract
Objectives:
Oral candidiasis is the most common oral infection that affects the oral mucosa. The most common oral thrush is caused by the fungus Candida albicans, but it can also be caused by Candida glabrata or Candida tropicalis. This study aimed to evaluate the antifungal effect of thymol-loaded chitosan nanocomposite in comparison with nystatin control drug on C. Albicans.
Material and Methods:
The obtained nanocomposite was characterized by scanning electron microscope (SEM), nanosizer-Zetasizer, and Fourier-transform infrared spectroscopy. Anti-Candida effects of thymol-loaded chitosan nanocomposite were assessed by evaluating the minimum inhibitory concentrations (MICs) and minimum fungicidal concentrations (MFCs) using broth microdilution method, according to the modified M60 protocol on yeasts, proposed by the Clinical and Laboratory Standards Institute.
Results:
Based on the results of SEM analysis, thymol-loaded chitosan nanocomposite with synthesized chitosan base shows a spherical shape. According to the size of the synthesized nanoparticles, the results showed that the size of nanoparticles varies from 100 to 600 nm, while most nanoparticles were between 200 and 300 nm with an average size of 295 nm. The lowest and the best MIC and MFC were related to the combination of nanoparticles + nystatin with 0.158 and 0.208 µg/ml, respectively. The results showed that the combination of nanoparticles + nystatin in comparison with nystatin group as a controlled drug showed a significant anti-Candida effect.
Conclusion:
The findings of the present in vitro study showed that thymol-loaded chitosan nanocomposite particularly along with nystatin showed promising antifungal effect against C. albicans as the main cause of oral candidiasis. Nevertheless, further investigations are required to elucidate the precise mechanism as well as systemic toxicity, especially in clinical settings.
Keywords
Oral candidiasis
Nanomedicine
In vitro
Treatment
INTRODUCTION
Candidiasis is one of the most important and common opportunistic fungal infections in humans, which is caused by some species of Candida, especially Candida albicans.[1] This opportunistic fungus can cause many clinical manifestations such as thrush, vaginitis, skin infection, endocarditis, meningitis, brain abscess, arthritis, and pyelonephritis.[2] In the human host, C. albicans is part of the natural flora of the mucosal surfaces of the oral cavity, gastrointestinal tract, and vagina; this fungus colonizes the mucosal surfaces and thus causes most endogenous infections from this site.[3] Oral candidiasis is the most common oral infection that affects the oral mucosa. The most common oral thrush is caused by the fungus C. albicans, but it can also be caused by Candida glabrata or Candida tropicalis.[4] Oral dentists often prescribe antifungal drugs such as nystatin or miconazole in the form of drops, gels, or sublingual tablets to treat the condition, known as oral thrush.[5]
In recent years, there have been numerous reports of failure to treat patients with various clinical forms of candidiasis; there are antifungal drugs with different formulations for treatment, which, in many cases, become recurrent due to the lack of proper response to the treatment of the disease, sometimes recurrences are observed.[6] In addition, the increased resistance of Candida species to antifungal drugs in patients with C. albicans-related diseases has led to the expansion of research and the use of antifungal drugs of natural origin.[7] In addition to the appropriate therapeutic effect, these compounds have had few adverse effects and some extracts of these plants have been promising in inhibiting the growth of various fungal species, including Candida.[8] Thymol (C10H14O) is a natural monoterpenoid phenol compound derived from herbal essential oils.[9] The results of experimental studies showed that thymol has various biological and medicinal properties such as antioxidant, antibacterial, antifungal, anti-cancer, anti-inflammatory, hepatosteroid, antispasmodic, and vasodilator properties.[10] Its antimicrobial effect is due to the increased permeability of the cell membrane by which it can chelate the surface cations of the membrane and interfere with vital activities.[11] One of the factors that limit the use of thymol is its low solubility in aqueous solutions, and this reduces its performance; therefore, to increase its efficiency, the use of biological nanocarriers is recommended.[12] Chitin is a natural polysaccharide and is prominent in crustaceans such as crabs and shrimp, insect cuticles, and fungal cell walls. Chitin and chitosan are widely used in medicine and industry as natural amino polysaccharides with unique structures and multifunctional properties. Their salient features include high biocompatibility, acceptable biodegradability along with low toxicity, as well as their antibacterial and anti-allergic properties.[13] This study aimed to evaluate the antifungal effect of thymol-loaded chitosan nanocomposite in comparison with nystatin control drug on C. albicans.
MATERIAL AND METHODS
Synthesis of nanocomposites
Chitosan solution (0.5%) was made in 20 ml of 1% acetic acid. One hundred mg of thymol was dissolved in dichloromethane and added dropwise to the chitosan solution under stirring. The solution was stirred for 2 hours. In another container, a 5% polyvinyl alcohol solution was prepared and a chitosan sample was gradually added. The final sample was dried in 8 cm plates at 40 °C.[14]
Determination of physical and chemical properties of nanoparticles
The produced nanoparticles were investigated using various instrumental methods. Nanoparticle images and their shape were prepared using scanning electron microscope (SEM). The particle size distribution of nanoparticles was obtained using laser beam diffraction method with nano-sizer-zetasizer and the functional groups on nanoparticles were determined using Fourier-transform infrared spectroscopy (FTIR).
Preparation of fungal suspension
In this study, the standard strain of C. albicans (ATCC5027) was used. To obtain uniform or homogeneous suspensions from fungal concentrations, a standard McFarland opacimeter with a degree of 0.5 was used, which contains a combination of barium chloride and sulfuric acid. Suspensions prepared with a dilution equivalent to the standard McFarland 0.5 diluted for C. albicans were estimated to contain approximately 103 cells. To prepare an inoculated suspension of yeast fungi, yeasts were first cultured in a suitable medium. After 18–24 h (logarithmic growth phase), it was removed from one of its colonies and transferred to a sterile tube containing 5 ml of physiological serum. It was then homogenized by a shaker and with the help of a spectrophotometer, at a wavelength of 530 nm and light transmittance of 90%, a yeast suspension with a cell density of 106 cells (CFU) per ml was obtained. In the broth microdilution method, a yeast suspension of 103 cells per milliliter was required, which was converted into a 5 cc sterile physiological saline tube and then 5 µL was removed and 5 µL of the yeast suspension was added. The resulting suspension contained 1 × 103 cells.[15]
Determination of minimum inhibitory concentration (MIC)
To determine the MIC of nanogels containing chitosan-based thymol-containing nanocomposite on standard strains of C. albicans, broth microdilution method was used in 96 sterile plates according to the Clinical and Laboratory Standards Institute (CLSI) instructions.[16] First, a stock of the CHT nanocomposite was prepared with Dimethyl sulfoxide (DMSO) solvent and sterile Müeller-Hinton broth culture medium, then, 50 µL of sterile Müeller-Hinton broth culture medium was added to the 3rd–12th rows. A 100 µL of stock was added to rows one and two, and dilution was performed from rows 2 to 10. In this way, from the second row, 50 µL to the third row and from the third row 50 µL to the fourth row, and up to the 10th row, dilution was done in the same way. Finally, from the 24 h culture of the desired microorganism at the rate of 50 µL equivalent to the semi-McFarland turbidity, 1.5×108 CFU/ml was added to rows 2–10. The plates were placed in a shaker incubator for 24 h at 35°C and then 2,3,5-triphenyltetrazolium chloride was used as a visual indicator for fungal growth. DMSO solvent was used for negative control and nystatin was used as the positive control; colorless wells were reported as MIC.
Determination of minimum fungicidal concentration (MFC)
After determining the MIC, the minimum dilution of the tube without growth turbidity, a loop of the contents of the thin tubes were transferred to plates containing solid culture and after 24–72 h of yeast growth or lack of growth was examined and the culture medium containing at least concentration of drug that lacks colony growth was determined as the minimum concentration of MFC.
Statistical analysis
All statistical tests are performed using SPSS software version 25.0. The independent t-test, analysis of variance (ANOVA), Tukey, and post hoc test are used to compare the results between groups. P < 0.05 will be considered a significant level.
RESULTS
SEM microscope analysis
Based on the results of SEM analysis, thymol-loaded chitosan nanocomposite with synthesized chitosan base shows a spherical shape [Figure 1]. According to the size of the synthesized nanoparticles, the results showed that the size of nanoparticles varies from 100 to 600 nm, while most nanoparticles were between 200 and 300 nm with an average size of 295 nm [Figure 2].

- Synthesized nanocomposite SEM microscope containing thymol-loaded chitosan nanocomposite.
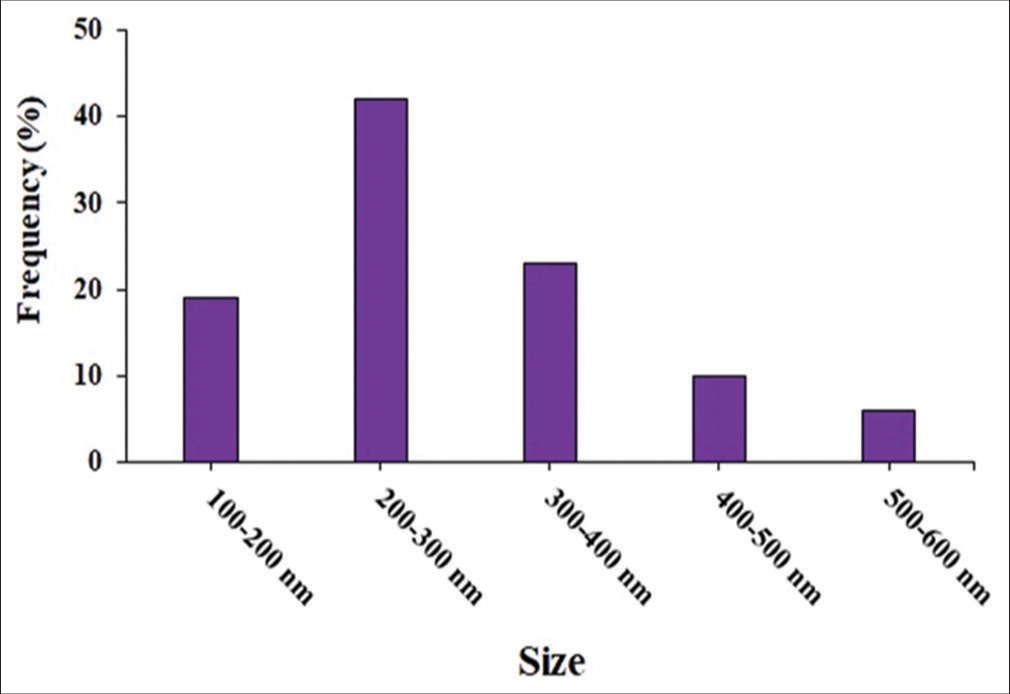
- Size distribution of thymol-based composite nanoparticles synthesized with chitosan.
FTIR analysis
Based on the results of FTIR analysis, the synthesized nanoparticles showed peaks at 3502, 2967, 1649, and 580 cm which can be related to the hydroxyl and primary amine group tension, C-H tension, amide tension, and ether bonds (C-O-C), respectively [Figure 3].

- FTIR spectrum of thymol-based composite nanoparticles synthesized with chitosan.
Determination of anti-Candida effect of nanocomposite by MIC and MFC assay
The anti-Candida effect of thymol nanocomposite containing chitosan on the standard strain of C. albicans was evaluated according to the CLSI standard method by calculating the MIC and MFC. The results after three repetitions for nanocomposites as well as nystatin as a controlled drug are shown in [Table 1]. The lowest and the best MIC and MFC were related to the combination of nanoparticles + nystatin with 0.158 and 0.208 µg/ml, respectively. The results showed that the combination of nanoparticles + nystatin in comparison with nystatin group as a controlled drug showed a significant anti-Candida effect.
| Drug | MIC (µg/ml) | MFC (µg/ml) |
|---|---|---|
| Thymol nanocomposite containing chitosan | 4.6±1.154 | 5.3±1.54 |
| Nystatin | 1.33±0.57 | 1.66±0.57 |
| Nanocomposite+nystatin | 0.158±0.079* | 0.208±0.072* |
DISCUSSION
Predisposing factors for oral candidiasis include changes in the microbial flora, use of broad-spectrum antibiotics, long-term use of mouthwashes, xerostomia, dentures and orthodontic appliances, use of corticosteroids, diseases and other conditions associated with the weakened immune system, smoking, poor oral hygiene, and pregnancy.[17] Commonly used antifungal agents are not only limited in number; rather, many of them are toxic and very expensive.[5] Recurrence of Candida infections is very common and this has increased the therapeutic burden of this opportunistic infection and consequently the need to develop new antifungal agents to expand the range of activities against Candida and to combat antifungal-resistant strains available.[18]
Nystatin, a macrolide produced by Streptomyces noursei, has excellent activity against Candida yeasts, especially C. albicans.[19] However, the bitter taste following the use of nystatin, the manifestation of allergic reactions after its use, and the possibility of adrenal insufficiency, liver necrosis, and drug interactions have been confirmed in many studies. Therefore, the problems associated with treating and managing Candida infections necessitate the discovery of new antifungal agents to broaden the range of activities against Candida.[19] Therefore, this study investigated the antifungal effect of chitosan-based thymol-containing nanocomposite in comparison with nystatin control drug on C. albicans.
The results of the present study showed that the nanoparticles were spherical and their size varied from 100 to 600 nm, while most nanoparticles were between 200 and 300 nm with an average size of 295 µm. According to the findings, the lowest MIC belonged to chitosan + nystatin-based thymol nanocomposite (0.158 µg/ml) and the highest amount belonged to chitosan-based thymol nanocomposite (4.6 µg/ml). Similarly, the composition of chitosan-based thymol nanocomposite with nystatin (0.208 µg/ml) and chitosan-based thymol nanocomposites alone (3.5 µg/ml) showed the lowest and highest MFC levels, respectively. The results of the present study showed that the composition of thymol nanocomposite based on chitosan + nystatin in comparison with nystatin had a significant anti-Candida effect and inhibited the growth and ultimately killed the fungus. In other words, the nanocomposite + nystatin combination exerts its inhibitory and lethal effect on C. albicans at lower concentrations than nanocomposite alone and nystatin alone. This means that chitosan-based thymol nanocomposite has increased the antifungal properties of nystatin.
Although the exact mechanisms of these compounds have not yet been elucidated, the previous studies have shown that these compounds can inhibit ergosterol formation by inhibiting effects on various enzymes responsible for ergosterol biosynthesis or exert their influence due to the increased permeability of the cell, which allows the passage of one or both antifungal agents.[20] Various mechanisms in the synergistic activity of antifungal agents such as chitosan-based thymol nanocomposites with nystatin can be explained, including the following: Inhibition of various stages in fungal intracellular pathways that are essential for cell survival; increased penetration of antifungal agent (nystatin) due to the effect of another antifungal agent (chitosan-based thymol nanocomposite) on the fungal cell membrane; inhibition of carrier proteins; and concomitant disruption of different cellular targets.[21]
Other studies on the antimicrobial effects of thymol have been performed: in the study of Shu et al. (2016), the in vitro antifungal effects of thymol on C. albicans were investigated as well as in vivo on Caenorhabditis elegans. They showed that thymol was able to inhibit the growth and biofilm formation of C. albicans; by increasing the expression of antimicrobial genes in the intestine, thymol increases the half-life of C. elegans at the time of infection and thus inhibits the formation of C. albicans biofilm.[22] Guo et al. (2009) showed that thymol had antifungal activity in vitro against susceptible and resistant clinical specimens of C. albicans. In addition, if thymol is combined with fluconazole or amphotericin, it shows more antifungal activity than when used alone.[23] In the study of De Castro et al. (2015), the antifungal activity and the effect of thymol and its synergy with nystatin against Candida species involved in oral infections were investigated. They reported that thymol provided an antifungal effect with a MIC of 39 µg/ml for C. albicans and C. krusei and 78 µg/ml for C. tropicalis. The combination of thymol and nystatin reduced the MIC values of both products by 84.4%. This study emphasizes that low concentrations of thymol are required to inhibit the growth of Candida strains and that the fungicidal effect of thymol is considered important for controlling infection; this is because the host’s immune responses are often compromised and make it difficult for infected patients to improve their health.[24]
Considering the mechanism of the action of thymol, the previous studies showed that thymol increases membrane permeability and can destroy the outer membrane of microorganisms, remove lipopolysaccharide, and increase cytoplasmic permeability to ATP.[25,26] In addition, the intracellular level of potassium ions decreased while the amount of this ion outside the cell increased intermittently; thymol appears to form channels throughout the membrane that allows potassium ions to leave the cytoplasm.[25,26] The results of the study by De castro et al. show that thymol acts on the surface of the fungal plasma membrane by interfering with the process of ergosterol biosynthesis, increasing membrane permeability, and reducing the components necessary for fungal cell survival; other mechanisms of action such as inhibition of spore germination, fungal proliferation, and cellular respiration may be involved.[24]
Natural products with inherent antimicrobial activity or products that enhance the activity of common antibiotics/antifungal agents may suggest new ways to control multidrug-resistant microorganisms and prevent them from coming into contact with synthetic products. Therefore, they reduce the risk of choosing new or improved resistance mechanisms.[27] According to the literature, there is scientific evidence for the use of nystatin in the treatment of fungal infections of the oral mucosa and it has been shown that it has advantages over other antifungals such as topical use and fewer side effects.[28] However, given the reported fungal resistance, the association between synthetic and natural antifungals could be an alternative to reducing the dose required for the effect and may reduce adverse side effects and prevent antifungal resistance. Hence, the findings of the present study support the development of clinical trials to evaluate the effectiveness of this combination therapy.[28,29]
CONCLUSION
The findings of the present in vitro study showed that thymol-loaded chitosan nanocomposite particularly along with nystatin showed a promising antifungal effect against C. albicans as the main cause of oral candidiasis. Nevertheless, further investigations are required to elucidate the precise mechanism as well as systemic toxicity, especially in clinical settings, is required.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Candidemia and invasive candidiasis in adults: A narrative review. Eur J Intern Med. 2016;34:21-8.
- [CrossRef] [PubMed] [Google Scholar]
- Candida and candidaemia. Susceptibility and epidemiology. Dan Med J. 2013;60:B4698.
- [Google Scholar]
- Candida and oral candidosis: A review. Crit Rev Oral Biol Med. 1994;5:125-57.
- [CrossRef] [PubMed] [Google Scholar]
- Current treatment of oral candidiasis: A literature review. J Clin Exp Dent. 2014;6:e576-82.
- [CrossRef] [PubMed] [Google Scholar]
- The antifungal effect of thymus vulgaris on isolated Candida albicans from the surface of removable orthodontic appliances. Herb Med J. 2019;4:55-64.
- [Google Scholar]
- The effects of nutraceuticals and herbal medicine on Candida albicans in oral candidiasis: A comprehensive review. Adv Exp Med Biol. 2021;1308:225-48.
- [CrossRef] [PubMed] [Google Scholar]
- Thymol bioactivity: A review focusing on practical applications. Arab J Chem. 2020;13:9243-69.
- [CrossRef] [Google Scholar]
- A review of therapeutic and pharmacological effects of thymol. Pharm Lett. 2016;8:150-4.
- [Google Scholar]
- The solid-phase of the dynamic microextraction of thymol and carvacrol using a porous, low-cost and unbreakable disk coated by polythiophene/multiwalled carbon nanotubes. Herb Med J. 2019;4:27-43.
- [Google Scholar]
- A comparison of chitosan gel and St. John's wort oil in second-degree burns: An experimental study. Herb Med J. 2021;6:18-26.
- [CrossRef] [Google Scholar]
- A comparison of the substrate specificities of endo-beta-N-acetylglucosaminidases from Streptomyces griseus and Diplococcus Pneumoniae. Biochem Biophys Res Commun. 1975;67:455-62.
- [CrossRef] [Google Scholar]
- Evaluation of the antifungal activities of various extracts from Pistacia atlantica Desf. Curr Med Mycol. 2015;1:25-32.
- [CrossRef] [PubMed] [Google Scholar]
- CLSI, Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi In: Approved Standard: CLSI document M38-A2. 2008.
- [Google Scholar]
- What makes oral candidiasis recurrent infection? A clinical view. J Mycol. 2014;2014:758394.
- [CrossRef] [Google Scholar]
- Efficacy of nystatin for the treatment of oral candidiasis: A systematic review and meta-analysis. Drug Des Dev Ther. 2016;10:1161-71.
- [CrossRef] [PubMed] [Google Scholar]
- Pharmacological properties and molecular mechanisms of thymol: Prospects for its therapeutic potential and pharmaceutical development. Front Pharmacol. 2017;8:380.
- [CrossRef] [PubMed] [Google Scholar]
- The synergistic antimicrobial effect of carvacrol and thymol in clay/polymer nanocomposite films over strawberry gray mold. LWT Food Sci Technol. 2015;64:390-6.
- [CrossRef] [Google Scholar]
- Thymol has antifungal activity against Candida albicans during infection and maintains the innate immune response required for function of the p38 MAPK signaling pathway in Caenorhabditis elegans. Immunol Res. 2016;64:1013-24.
- [CrossRef] [PubMed] [Google Scholar]
- Antifungal activity of thymol against clinical isolates of fluconazole-sensitive and-resistant Candida albicans. J Med Microbiol. 2009;58:1074-9.
- [CrossRef] [PubMed] [Google Scholar]
- Antifungal activity and mode of action of thymol and its synergism with nystatin against Candida species involved with infections in the oral cavity: An in vitro study. BMC Complement Altern Med. 2015;15:417.
- [CrossRef] [PubMed] [Google Scholar]
- A brief perspective on anti-inflammatory effects of thymol and carvacrol. Herb Med J. 2017;2:137-8.
- [Google Scholar]
- Thymol and thyme essential oil-new insights into selected therapeutic applications. Molecules. 2020;25:E4125.
- [CrossRef] [PubMed] [Google Scholar]
- Synergistic interactions of phytochemicals with antimicrobial agents: Potential strategy to counteract drug resistance. Chem Biol Interact. 2019;308:294-303.
- [CrossRef] [PubMed] [Google Scholar]
- Essential oils and their natural active compounds presenting antifungal properties. Molecules. 2019;24:E3713.
- [CrossRef] [PubMed] [Google Scholar]
- Synthesis, antifungal activity of caffeic acid derivative esters, and their synergism with fluconazole and nystatin against Candida spp. Diagn Microbiol Infect Dis. 2016;86:387-91.
- [CrossRef] [PubMed] [Google Scholar]






