Translate this page into:
New Drug Therapies for Women with Eclampsia – Impact on Prognosis as Compared to Standard Treatment: A Systematic Review of Randomized Controlled Trials

*Corresponding author: Indrajit Banerjee, Department of Pharmacology, Sir Seewoosagur Ramgoolam Medical College, Belle Rive, Mauritius. indrajit18@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Reechaye D, Perrine AA, Robinson J, Banerjee I. New Drug Therapies for Women with Eclampsia – Impact on Prognosis as Compared to Standard Treatment: A Systematic Review of Randomized Controlled Trials. Glob J Med Pharm Biomed Update. 2024;19:10. doi: 10.25259/GJMPBU_5_2024
Abstract
Hypertensive disorders continue to be a major global health concern, impacting millions of pregnant women annually, and have a significant impact on both the mortality and morbidity rates of mothers and neonates. The two major spectrums of disease are pre-eclampsia (PE) and eclampsia. While pre-eclampsia (PE) is characterized by milder symptoms, eclampsia includes hypertensive symptoms plus impaired brain function and seizures or coma. This systematic review aims to explore safer and novel drugs/drug combinations with minimal adverse effects to the mother and fetus, which will cause a rapid decrease in blood pressure, better bioavailability, and prevent life-threatening complications. The PubMed and PubMed Central databases were used to perform a comprehensive literature review from January 2019 to December 2023. The Mesh terms and Boolean operators used were “Pre-eclampsia” OR “Eclampsia” AND “Drug therapy.” There are novel and promising alternatives to mono-therapeutic magnesium sulfate in the treatment of severe PE and eclampsia. These drugs are namely dexmedetomidine, nifedipine in combination with phytosterol, magnesium sulfate, and compound Danshen combinations. The use of these drugs is, however off-label in nature and should be instituted solely on a case-by-case basis at the discretion of the physician. The prospect of a combination therapy being developed from the above drug groups is promising, and these findings should instigate further research to provide the next generation of pharmacotherapeutic treatment protocols to combat severe PE and eclampsia.
Keywords
Pre-eclampsia
Eclampsia
Drug therapy
Hypertension
Pregnancy-induced
INTRODUCTION
Hypertensive disorders continue to be a major global health concern, impacting millions of pregnant women annually, and have a significant impact on maternal and neonatal mortality and morbidity. The two main spectrums of disease are pre-eclampsia (PE) and eclampsia. While PE is characterized by milder symptoms, eclampsia includes hypertensive symptoms plus impaired brain function and seizures or coma.[1,2]
Eclampsia usually manifests as high blood pressure (BP), proteinuria, and edema, which occurs after 20 weeks of gestation.[3,4] It affects multiple systems of the body, which account for its fatality through irreversible damage to the mother’s organs, endangering both maternal and fetal life.
Despite a global decline of 34.2% in the maternal mortality ratio (MMR) from 2000 to 2020, MMR is still a great challenge in the African region.[5] According to the World Health Organization (WHO) (iAHO Maternal Mortality Regional Factsheet 2023), hypertensive disorders account for 22.0% of maternal mortality, which is 2nd most common cause of death after hemorrhage.[5]
The pathophysiology of this disorder remains obscure. The theories put forward more commonly involve vascular injury. Endothelial activation and endothelial cell dysfunction are the hallmark of PE and are, therefore, one of the most important etiopathologies.[6]
The management of this obstetric condition involves drug treatments aiming at the rapid control of BP and prevention of severe complications until the fetus reaches an appropriate gestational age for delivery. The standard drug therapy mainly includes antihypertensives and magnesium sulfate.[7] However, these drugs bear adverse effects and are sometimes not as effective as they are claimed to be. This is the driver for further studies to be undertaken to delineate which treatments will best prevent unfavorable outcomes related to hypertension (HTN) during pregnancy. This systematic review was conducted to identify drugs for better prevention and better treatment of the condition by providing tailor-made therapy to those suffering from the disorder. This research is not necessarily designed to find better and novel drugs but to find regimens and combinations that improve the standard tried and tested drugs currently in use. This can be achieved by combining standard drugs in use with adjuvant drugs that potentiate their effect[8] and, in other instances, reduce the risk of systemic toxicity.[2] Certain drugs under study may show the ability to bring about protective effects on the respective damaged organs in parturient with eclampsia. The effectiveness of the drug is expressed through levels of biological markers in those individuals.[9] While the disease outcome is directly related to early diagnosis and management, it is obvious that more consideration should be put on those new drugs/drug combinations that are not used as standard treatment.[10-12] This systematic review aims to explore safer and novel drugs/drug combinations with minimal adverse effects to the mother and fetus, which will cause a rapid decrease in BP, better bioavailability, and prevent life-threatening complications.
METHODOLOGY
The PubMed and PubMed Central databases were used to perform a comprehensive literature review. The Mesh terms and Boolean operators used were “Pre-eclampsia” OR “Eclampsia” AND “Drug therapy.” This study is compliant with PRISMA guidelines, ensuring transparency and adherence to recognized standards.
Inclusion criteria
The articles included in the study were all randomized control trials. Full-text articles published in English between January 2019 and December 2023 were chosen. Articles in this study addressed drugs/drug combinations that were novel and used to treat patients suffering from PE. The new drugs/drug combinations included proved to be efficient in managing the raised BP and avoiding the consequent complications of PE and eclampsia.
Exclusion criteria
Abstracts, cross-sectional studies, cohort studies, case series, case studies, reviews, systematic reviews, and letters to the editor were excluded from this study. Articles that highlighted the effectiveness of supplements that are generally prescribed in normal pregnancy but which can also contribute to preventing PE were excluded since they were not novel drugs.
Articles that focused solely on standard drugs such as labetalol and hydralazine, which are regularly used in the management of PE and eclampsia, were not included in the study.
Data extraction
Relevant titles and abstracts were screened in PubMed and PubMed Central. All the randomized controlled trials which met the eligibility criteria were selected for the evaluation. All of the literature surveys were independently conducted by independent researchers (DR and ALAP). The data extraction tables included the author, year of study, study design, number of intervention patients, the control group (CG) of patients, both the inclusion criteria, drug/combination of drug(s), therapeutic protocol, major findings, outcome of therapy, and potential limitations.
Quality assessment
Quality assessment was performed using the 25-item checklist CONSORT to assess the methodological quality of the studies that met the inclusion criteria of the randomized controlled trials.
RESULTS
The systematic literature search performed resulted in a total of 4,360 articles, of which 4,032 were earmarked as irrelevant and excluded from the primary screening. A further rigorous evaluation and analysis of the relevant titles as well as abstracts based on both the inclusion and exclusion criteria ultimately resulted in the inclusion of 6 randomized controlled trials from the analysis of the quality assessment [Figure 1].
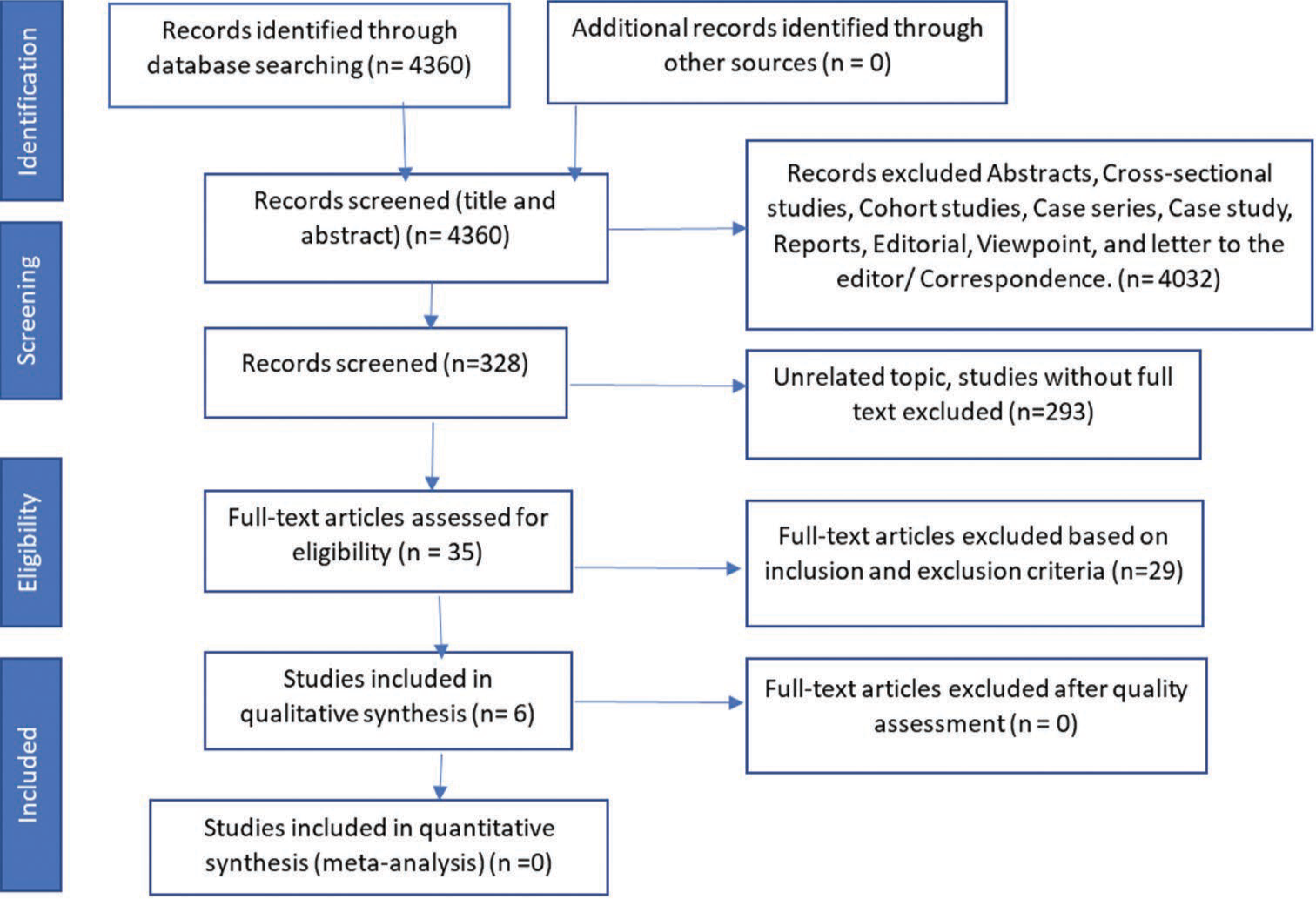
- Inclusion of articles by (PRISMA) preferred reported items for systematic reviews. PRIMSA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
The systematic review revealed some new drugs and new drug combinations that have proved to be beneficial in the treatment of PE and eclampsia. Table 1 depicts summary of studies, sample size and inclusion criteria. In addition, they have also each shown to have particular advantage(s) over at least one of the standard drugs currently being used [Table 2].
| Author, year | Study design | No. of intervention patients | The control group of patients | Inclusion criteria |
|---|---|---|---|---|
| Zhou, et al. 2022.[3] | Randomized Control Trial | 30 | 30 | Inclusion criteria: Fits diagnostic criteria of moderate-to-severe PE. PE in OBG, no previous history of PE, gestational age of onset<34 weeks, and gestational age of delivery was between 28 and 33 weeks, no prior treatment before admission, full participation of patient and consent. |
| Zhang and Feng, 2019[8] | Randomized Control Trial | 127 | 126 | Single pregnancy with mothers aged between 25 and 35 who were diagnosed with severe PE in the Liaocheng People’s Hospital. |
| Zhang, et al. 2019.[9] | Randomized Control Trial | 67 | 67 | Patients who have met the PE diagnostic criteria with cesarean section indications; 18–40-year old with a gestation above 34 weeks; SBP >140 but under 160 mmHg and DBP higher than 90 but below 110 mmHg; urinary protein dipstick 1+or 2++. |
| Yu, et al. 2019.[10] | Randomized Control Trial | 40 | 40 |
|
| Karemore and Avari, 2019.[11] | Randomized control trial | 5 | 5 | Ten healthy female volunteers with a mean age of 24 years (the range 22–27 years), a mean weight of 60 kg (a range between 57 and 65 kg), and a mean height of 152 cm (range 149–163) were enrolled for the study. |
| Furuhashi, et al., 2021.[12] | Randomized control trial: Phase II | 12 | 14 | Singleton pregnancies with a raised DBP between 20 and 33 weeks of gestation. |
PE: Preeclampsia, SBP: Systolic blood pressure, DBP: Diastolic blood pressure, OBG: Obstetrics and gynaecology
| Author, year | Drug/combination of drug (s) | Therapeutic protocol | Main findings | Outcome of therapy | Potential limitations |
|---|---|---|---|---|---|
| Zhou, et al. 2022.[3] | Compound Danshen injection combined with magnesium sulfate | The CG received the conventional regimen of 5 g magnesium sulfate, which was dissolved in 20 mLs of a 5% glucose solution and infused over a 30 min period. The IG was treated with compound Danshen injection in a dosage of 16 mL in addition to the above protocol received by the CG. | IG: The BP (SBP and DBP) decreased significantly. Also, TNF-α, D-D, Fib, and 24 h urinary protein in this group decreased more after treatment than the CG. NO level increased more than in CG. |
The compound Danshen injection adjuvant therapy was shown to optimize the clinical manifestations of severe PE and reduce systemic hypercoagulability. It also improves the liver and kidney function. | There was an insufficient sample size. A multi-center trial on a larger scale should be conducted. |
| Zhang and Feng, 2019[8] | Nifedipine, along with phytosterol | Nifedipine and phytosterol group were administered nifedipine capsules in a dosage of 10 mg, and up to five dosages with up to 5 doses and phytosterol capsules were administered in 300 mg dosages with a max of 5 dosages every 15 min until the BP was reduced to <150/100 mmHg. Nifedipine and placebo group were administered nifedipine capsules with 10 mg in each dosage and up to a total of 5 doses. This was administered with a placebo of starch capsules (each dose being 300 mg and a maximum of up to five doses) every 15 min until the BP was reduced to <150/100 mmHg. | IG: Was found to require a lower dosage for the control of their BP. Presented with a shorter time for the control of the BP (41.1±13.5 min) as opposed to the nifedipine and placebo group (53.7±18.2 min). Exhibited a remarkably delayed time for the occurrence of another hypertensive crisis (8.3±1.7 h) compared to nifedipine+placebo (6.1±1.9 h). |
Phytosterol obviously improved the treatment outcome of HTN recorded in PE during pregnancy. | Phytosterols MOA on the control of HTN remains unknown. MMP-3 and MMP-13 have been found to be implicated, but it needs to be confirmed. A multicenter trial on a larger scale is warranted. |
| Zhang et al. 2019.[9] | Dexmedetomidine | The IG was administered dexmedetomidine intravenously at a dosage of 0.4 μg/(kg•min) for 10 min before surgery, whereas the CG received 0.9% of NS over a 10 min period. | Both SBP and DBP decreased more in the IG compared to the CG. After 24 h: CG: SBP=172.4±12.2 mmHg, DBP=97.0±14.2 mmHg IG: SBP=124.9±11.6 mmHg, DBP=70.5±7.1 mmHg Compared with the CG, the levels of β2-MG, KIM-1, and urine protein dropped more significantly at each time point after administration of dexmedetomidine. |
Dexmedetomidine improved the symptoms of PE and aids in the down-regulation of the levels of β2-MG, KIM-1, and urine protein, thereby exerting a protective effect on kidney injury in pre-eclamptic parturients. | A larger number of experiments should be conducted in order to better understand the underlying mechanism of action of dexmedetomidine. |
| Yu, et al. 2019.[10] | Ulinastatin | Ulinastatin (5000 IU/kg) diluted in 20 mL of NS was administered intravenously into parturients within a 5 min window. The same volume of NS was injected intravenously into the CG. 5 mL of blood was collected either before anaesthesia and/or 40 min after ulinastatin or saline administration; the following were measured: -The plasma concentration of vWF. -The platelet count and hemoglobin levels. -PT, APTT, and fibrinogen concentration. |
40 minutes after the administration of ulinastatin in IG: The plasma concentration of vWF decreased significantly (1330±515 U/I) compared to baseline levels (1722±556 U/I) and to the CG (1719±543 U/I). APTT was prolonged (30.6±1.8 s) compared to the baseline levels (27.3±2.0 s) as well as to the CG (27.5±1.9 s). | Ulinastatin is believed to alleviate vascular endothelial cell injury in pregnant women with severe PE after a cesarean section. | This was a single-centre study, where ulinastatin was only administered once, and the overall study was of short duration. A long-term, multicenter clinical trial is needed. |
| Karemore and Avari, 2019.[11] | Gastroretentive drug delivery system of nifedipine | The CG was administered t (Nicardia XL) while the (IG) received the optimized formulation. Hematological samples were withdrawn and at the following intervals collected at (0.5 h, 1 h, 2 h, 3 h, 4 h, 5 h, 6 h, 7 h, 8 h, 9 h, 10 h, 11 h, and 12 h) after the drug administration. |
A significant effect on the drug release at the 3-hour, 6-hour, and 12-hour intervals were noted in the IG. | The GRDDS of nifedipine greatly improves the oral bioavailability of the drug. | This new formulation may be costly and not affordable to all. |
| Furuhashi, et al. 2021.[12] | Tadalafil | The treatment group received a 20 mg oral tadalafil dosage daily. No drug was taken by the conventional treatment group. | CG: SBP= 146.0–169.0 mmHg DBP=90.0–101.0 mmHg Headaches: 43% IG: SBP=144.0–161.0 mmHg DBP=88.0–102.0 mmHg Headaches: 0% | Maternal headache was significantly decreased. Tadalafil provides vascular protection to the endothelium through its vasodilating action. | A small participant cohort. This study was an open-label trial. It was interrupted by recommendations from the PMDA and could not be accomplished. |
HTN: Hypertension, BP: Blood pressure, SBP: Systolic blood pressure, DBP: Diastolic blood pressure, NS: Normal saline, PT: Prothrombin time, h: hours, APTT: Activated partial thromboplastin time, IG: Interventional group, CG: Control group, β2-MG: β2-microglobulin, KIM-1: Kidney injury molecule-1, TNF-α: Tumor necrosis factor alpha, PE: Pre-eclampsia, IG: Intervention group, MOA: Mechanism of action, MMP: Matrix metalloproteinase, vWF: von Willebrand factor, CG: Control group, GRDDS: Gastro retentive drug delivery system, PMDA: Pharmaceuticals and Medical Devices Agency, NO: Nitric oxide
DISCUSSION
PE and eclampsia are often the cause of sleepless nights for many obstetricians, as these hypertensive conditions are often difficult to manage. The treatment modalities for these hypertensive conditions range from conservative lifestyle and dietary measures to aggressive pharmacotherapeutic and surgical measures. The use of drugs in treating PE is a necessity and is often a lifesaving preventative measure to retard the development of eclampsia. Magnesium sulfate is the drug most often initiated for both eclampsia and severe PE, and according to the WHO, it is the drug of choice in such conditions.[13] Many doctors, however, disagree with the current dosing regimen thereof. A review study performed by Pratt et al. on 248 fully reviewed studies concluded that lower dosages of magnesium sulfate may be as effective as traditional therapeutic dosing. Magnesium sulfate is believed to have a high rate of catastrophic side effects such as hyporeflexia, confusion, hypotension, muscle weakness, respiratory depression, and renal insufficiency. It is thus logical that an alternate drug or class of drugs are further studied and implemented in the treatment of these hypertensive conditions, which will produce the same desired result without the negative side effects produced by magnesium sulfate.[14]
Some of the newer drug alternatives have shown the potential to be more effective than magnesium sulfate in controlling HTN. Two strong alternatives to magnesium sulfate are dexmedetomidine and a nifedipine and phytosterol combination. The difficulty in gaining approval for such drugs is to exclude potential teratogenicity, thereby requiring intense and prolonged pharmacovigilance. Dexmedetomidine is a promising drug which may be used as an alternative to labetalol in hypertensive asthmatic patients. In a randomized control trial performed by Zhang et al., on 134 patients, it was found that both the systolic BP and diastolic BP decreased more in the interventional group (IG) receiving the dexmedetomidine as opposed to the placebo. The reduction in the urinary concentrations of β2-microglobulin, kidney injury molecule-1, and urine protein post infusion suggests that the drug has a significant reno-protective effect. The mechanism of action of dexmedetomidine is not well understood, and further studies will need to be undertaken to ensure that long-term exposure to the drug poses no harm to both the mother and unborn child.[9] A further study performed by Yu et al. concluded that the use of dexmedetomidine in patients with gestational HTN after cesarean sections can enhance the sedative effect as well as aid in the recovery of the postpartum mothers, protect the myocardium, reduce the “stress response,” and enhance coagulation thereby promoting the use of this drug.[15]
Another therapeutic alternative is to use traditional drugs such as calcium channel blockers, for example, nifedipine, in combination with adjuncts such as phytosterol. A randomized control trial was performed by Zhang and Feng, on 253 patients. The CG received 10 mg nifedipine tablets with a starch placebo, and the IG received 10 mg nifedipine tablets with 300 mg phytosterol capsules. The IG displayed a notable delayed time for the re-occurrence of another hypertensive crisis (8.3 ± 1.7 h as compared to the nifedipine placebo (6.1 ± 1.9 h). It was also noted that a lower dosage of the drugs was required to control the HTN in the IG. Phytosterol still poses a challenge in that its mechanism of action is unknown, and it is evident, however, that a multi-center trial in a larger center is warranted.[8] An additional study performed by Gao et al., on phytosterol in pregnancy, with women suffering from gestational diabetes concluded that their study supported the potential clinical efficacy of phytosterol in alleviating both maternal symptoms and neonatal complications of mothers suffering from gestational diabetes. It is thus vital for more studies to be undertaken to understand the full potential of phytosterol.[16]
A combination of magnesium sulfate and compound Danshen was trialed by Zhou et al., and the CG received a conventional treatment of 5 g of magnesium sulfate, which was dissolved in 20 mL of 5% glucose solution and was intravenously infused in a 30-min window.[3] The IG received the additional compound Danshen injection (16 mL). The compound Danshen injection adjuvant therapy was found to optimize the clinical manifestations of S-PE and reduce systemic hypercoagulability. It was also found to improve the kidney function.[2] It is suggested that this study should be repeated with a lower dose of magnesium sulfate as if the Danshen adjuvant can still produce similar results; despite the lowered magnesium sulfate dosage, it would reduce the likelihood of magnesium sulfate-induced side effects and could thereby be a viable option for the treatment of PE and eclampsia. A meta-analysis performed by Chen et al., on the combined efficacy of Danshen and conventional antihypertensives in the treatment of hypertensive left ventricular hypertrophy (HLVH), concluded that the antihypertensive effect of a drug combination with Danshen and conventional anti-hypertensives could be greater and more beneficial for the patients. This study further supports the notion that Danshen, in combination with magnesium sulfate, may provide a greater pharmaceutical outcome and benefit as opposed to monotherapy.[17]
It is only after the drugs discussed above are recognized and approved by the relative bodies that the information and data provided should sensitize clinicians to the alternate possibilities available to treat PE and eclampsia. The decision to select an alternate drug should strictly be made on a case-by-case basis and should be determined by the relative indications and contraindications of the specific patient.
CONCLUSION
There are novel and promising alternatives to mono-therapeutic magnesium sulfate in the treatment of severe PE and eclampsia. These drugs are namely dexmedetomidine, nifedipine in combination with phytosterol, magnesium sulfate, and compound Danshen combinations. The use of these drugs is, however, off-label in nature and should be instituted solely on a case-by-case basis at the discretion of the physician. The prospect of a combination therapy being developed from the above drug groups is promising, and these findings should instigate further studies to provide the next generation of pharmacotherapeutic treatment protocols to combat severe PE and eclampsia.
Acknowledgment
We extend our appreciation to Honorable Chairman RPN Singh and Prof. Dr. AP Singh (principal In-charge) Sir Seewoosagur Ramgoolam Medical College, Belle Rive, Mauritius for constant encouragement and support to conduct the study. The authors are also thankful to Mr. Jeenarain Tusharsingh and Ms. Deshna Bundhun, Final Year Medical Students at Sir Seewoosagur Ramgoolam Medical College, Belle Rive, Mauritius for their generous help in conducting this research.
Authors contribution
Driti Reechaye, Anne Laure Annaïck Perrine, Jared Robinson and Indrajit Banerjee have contributed equally in this Research and should be considered as primary co-first authors for this research paper.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
Patient consent is not required as there are no patients in this study.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- PreEclampsia: Pathogenesis, Novel Diagnostics and Therapies. Nat Rev Nephrol. 2019;15:275-89.
- [CrossRef] [PubMed] [Google Scholar]
- Preeclampsia Diagnosis and Management. Best Pract Res Clin Anaesthesiol. 2022;36:107-21.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of Compound Danshen Injection Combined with Magnesium Sulfate on Oxidative Stress, TNF-a, NO, and Therapeutic Efficacy in Severe Preeclampsia. Comput Intell Neurosci. 2022;2022:9789066.
- [CrossRef] [PubMed] [Google Scholar]
- Postpartum Preeclampsia or Eclampsia: Defining its Place and Management among the Hypertensive Disorders of Pregnancy. Am J Obstet Gynecol. 2022;226:S1211-21.
- [CrossRef] [PubMed] [Google Scholar]
- Maternal Mortality: The Urgency of a Systemic and Multisectoral Approach in Mitigating Maternal Deaths in Africa. 2023. Analytical FACT Sheet. Available from: https://files.aho.afro.who.int/afahobckpcontainer/production/files/iaho_maternal_mortality_regional_factsheet.pdf [Last accessed on 2024 Feb 26]
- [Google Scholar]
- Pathophysiology of Preeclampsia: An Angiogenic Imbalance and Long-Lasting Systemic Vascular Dysfunction. Hypertens Res. 2017;40:305-10.
- [CrossRef] [PubMed] [Google Scholar]
- O60. Treatment of Hypertension in Pregnancy. Abstracts/Pregnancy Hypertension. Pregnancy Hypertens. Int J Women's Cardiovasc Health. 2015;5:209-58.
- [CrossRef] [Google Scholar]
- Phytosterol Enhances Oral Nifedipine treatment in Pregnancy-Induced Preeclampsia: A Placebo-Controlled, Double-Blinded, Randomized Clinical Trial. Exp Biol Med (Maywood). 2019;244:1120-4.
- [CrossRef] [PubMed] [Google Scholar]
- Protective Effect of Dexmedetomidine on Kidney Injury of Parturients with Preeclampsia Undergoing Cesarean Section: A Randomized Controlled Study. Biosci Rep. 2019;39:BSR20190352.
- [CrossRef] [PubMed] [Google Scholar]
- Ulinastatin Attenuates Vascular Endothelial cell Damage in Pregnant Women with Severe Pre-Eclampsia. Acad Bras Cienc. 2019;91:e20180746.
- [CrossRef] [PubMed] [Google Scholar]
- Formulation, Optimization, and in Vivo Evaluation of Gastroretentive Drug Delivery System of Nifedipine for the Treatment of Preeclampsia. AAPS Pharm Sci Tech. 2019;20:200.
- [CrossRef] [PubMed] [Google Scholar]
- Tadalafil Treatment for Preeclampsia (Medication in Preeclampsia; MIE): A Multicenter Phase II Clinical Trial. J Matern Fetal Neonatal Med. 2021;34:3709-15.
- [CrossRef] [PubMed] [Google Scholar]
- Alternative Regimens of Magnesium Sulfate for Treatment of Preeclampsia and Eclampsia: A Systematic Review of Non-Randomized Studies. Acta Obst Gynecol Scand. 2016;95:144-56.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of Dexmedetomidine on Myocardial Protection and Changes of Homocysteine and D-Dimer in Patients with Gestational Hypertension after Cesarean Section. Int J Clin Exp Med. 2020;13:5406-12.
- [Google Scholar]
- Phytosterol Nutritional Supplement Improves Pregnancy and Neonatal Complications of Gestational Diabetes Mellitus in a Double-Blind and Placebo-Controlled Clinical Study. Food Funct. 2017;8:424-8.
- [CrossRef] [PubMed] [Google Scholar]
- Meta-Analysis of Clinical Efficacy and Safety of Compound Danshen Dripping Pills Combined with Conventional Antihypertensive Drugs in Treatment of Hypertensive Left Ventricular Hypertrophy. Zhongguo Zhong Yao Za Zhi. 2021;46:2578-87.
- [Google Scholar]







