Translate this page into:
Beyond the Antidepressant Action, Paroxetine in Managing the Hot Flashes in Women with Menopause: A Systematic Review

*Corresponding author: Dr. Ajinkya Sureshrao Ghogare, Assistant Professor, Department of Psychiatry, Government Medical College, Akola, Maharashtra, India. ajinkyaghogaremd@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Ghogare AS, Talhan TS, Madavi PB, Joshi AC, Telgote SA, Ambad RS. Beyond the Antidepressant Action, Paroxetine in Managing the Hot Flashes in Women with Menopause: A Systematic Review. Glob J Med Pharm Biomed Update. 2023;18:31. doi: 10.25259/GJMPBU_38_2023
Abstract
Background:
Women in the menopausal phase of their lives often experience the vasomotor symptoms of menopause, namely, hot flushes or flashes and disturbances of sleep. About 75–85% of menopausal women tend to experience one or more vasomotor symptoms of menopause. Menopausal hormone therapy (MHT) is considered to be the mainstay treatment in treating vasomotor symptoms of menopause. However, MHT tends to be accompanied by adverse outcomes and there exist contraindications to it. Hence, an alternative treatment strategy is required in view of contraindications, intolerance, or side effects of MHT. Recently, paroxetine is the first and only selective serotonin reuptake inhibitor antidepressant which is United States Food and Drug Administration approved as a non-hormonal management method of vasomotor/climacteric symptoms in menopausal women.
Objective:
In the present study, we systematically reviewed paroxetine’s role in the management of hot flashes in menopausal women.
Material and Methods:
For the review purpose, we included the previously published relevant original, review, meta-analysis, and randomized controlled trial articles that were published in the English language using a 4-phase process of the preferred reporting items for systematic reviews and meta-analyses statement.
Results:
The severity and frequency of hot flashes were significantly reduced among menopausal women who received paroxetine compared to placebo. Five studies also showed improvement in the night-time sleep duration among menopausal women who received paroxetine in low doses.
Conclusion:
Thus, this study shows that low-dose paroxetine can be a beneficial and effective non-hormonal management option in managing hot flashes among menopausal women.
Keywords
Paroxetine
Menopause
Climacteric symptoms
Hot flashes
Hot flushes
Sleep disturbances
INTRODUCTION
Menopause
Menopause or climacteric period is a usual, non-pathological state in the life of women that is characterized by cessation of the menses over the period of at least 1 year.[1] It routinely occurs among all menstruating women as a result of age-related estrogen deficiency near about 51 years of median age.[1] Although considered a non-pathological condition, about 85% of menopausal women may exhibit various range of symptoms such as vasomotor symptoms, urogenital symptoms, cardiovascular symptoms, and psychological issues such as insomnia, mood fluctuations, and cognitive changes.[1-4] Women tend to spend about a third part of their lifespan in the period of postmenopause.[1]
Epidemiology of menopause
Globally, around 50 million women tend to enter the menopausal phase of their lives annually.[5] Naturally, menopause occurs between the ages of 45–55 years globally.[6] Around 5% of women move into menopause or the climacteric phase between the ages of 40–45 years.[1] Around 1% of females tend to encounter menopause prematurely before 40 years of age, which may be secondary to permanent failure of ovaries due to abnormalities of sex chromosomes, autoimmune disorders, or other unknown reasons.[1,6]
Etiology of menopause
As a woman ages, the number of ovarian follicles tends to diminish. Reduction in the granulosa ovarian cells leads to declined amounts of inhibin hormone and estradiol hormone which subsequently leads to increased synthesis of the luteinizing hormone and the follicle-stimulating hormones (FSH) due to loss of inhibition on gonadotropins from the reduced levels of both estradiol and inhibin.[1] This leads to the disturbance of the hypothalamic-pituitary ovarian axis causing failure in the proliferation of endometrium and irregularities in a menstrual cycle until the menses stop.[1] [Figure 1] beside summarizes the age-related non-pathological etiology of the menopause.
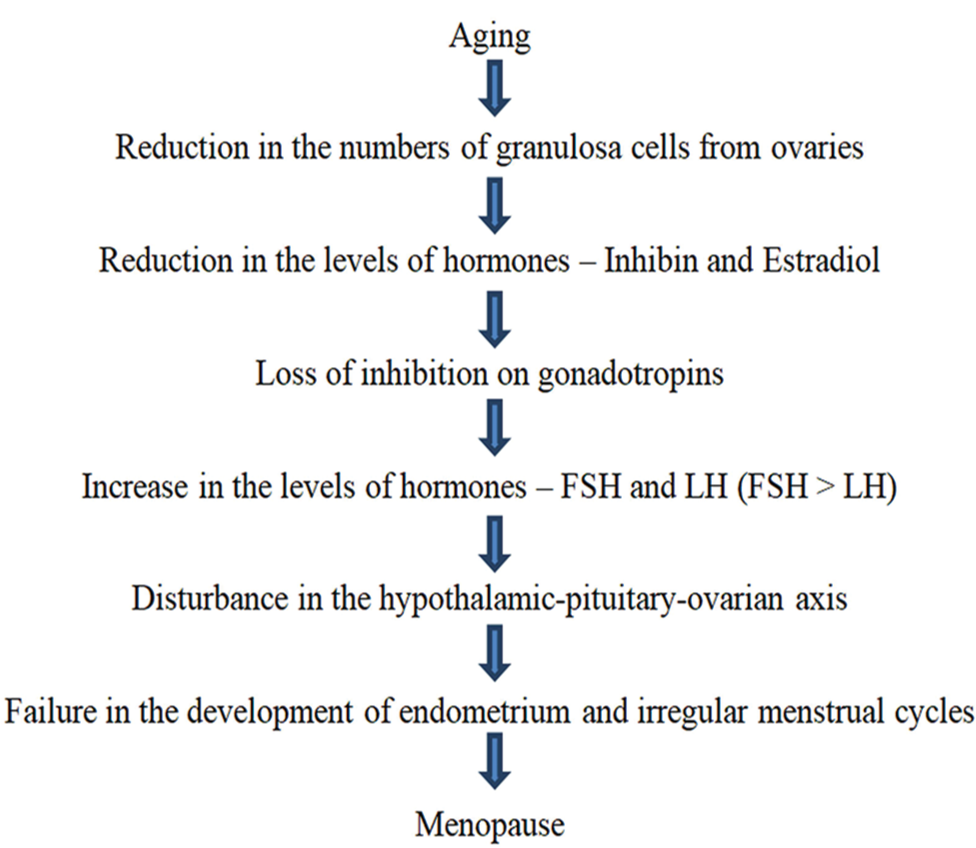
- Normal age-related non-pathological etiology of menopause.
Menopause may occur secondary to breast cancer treatment and endometriosis with antiestrogens as well as chemotherapy medicines.[1] Surgical interventions such as bilateral removal of ovaries and hysterectomy tend to cause menopause.[1]
Vasomotor symptoms of the menopause
About 75–85% of menopausal women experience various vasomotor or climacteric symptoms that include hot flushes/flashes, palpitations, migraines, and night sweats.[1,2] Vasomotor symptoms of menopause tend to last about 5 years on average.[7] About 55% of premenopausal women tend to experience hot flashes even before the onset of menstrual irregularities which indicate transition to the menopausal phase.[8] Episodes of hot flashes tend to last for 3–4 min at unforeseeable intervals.[1] Hot flashes can be exacerbated by various factors such as emotional stress, alcohol, eating, and exertion.[1] The precise etiology of hot flushes/flashes is unclear, but it is postulated that narrowing and re-setting of a thermoregulatory system of the body due to changing estrogen levels causes hot flushes/flashes in menopausal females.[2] Fluctuating levels of hormone estradiol, reduced inhibin B levels, and raised levels of FSH were found to be linked with the occurrence of vasomotor symptoms including hot flashes.[9] [Figure 2] below summarizes the mechanism of occurrence of hot flushes/flashes among menopausal females.[2,10]
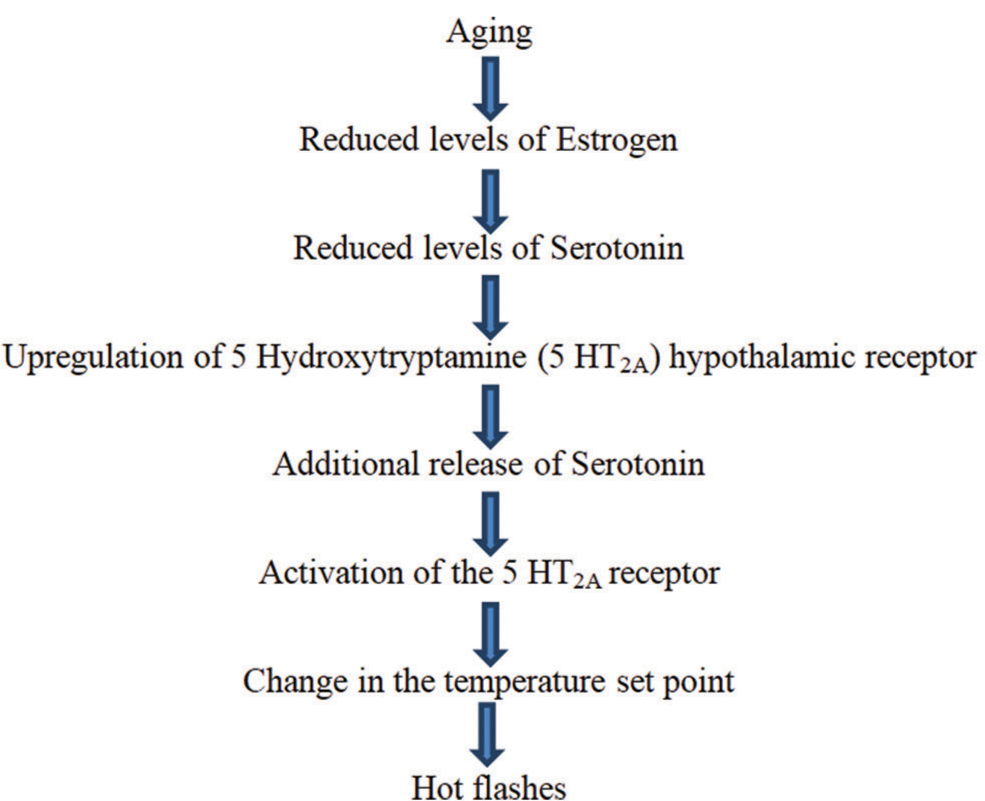
- Mechanism of occurrence of hot flashes in menopausal women.
Other vasomotor symptoms include migraine headaches. Intensity as well as severity of migraine episodes tend to fluctuate. Migraines with aura are less prevalent than those without aura.[1] Other menopausal symptoms include urogenital and psychological symptoms.
Urogenital or vulvovaginal symptoms of menopause
About 60% of menopausal women experience various urogenital symptoms such as urethral atrophy and vaginal atrophy.[1] Urethral atrophy can result in various issues such as urinary urgency, frequency, dysuria, and stress incontinence. Vaginal atrophy may present as dyspareunia, decline in libido, vaginal dryness, and pruritus.[1] Urogenital tissues are estrogen sensitive and about 27% of menopausal women experienced vaginal dryness, 18.6% experienced vaginal itching, 11.1% experienced vaginal discharge, and 5.2% experienced dysuria.[11] Vulvovaginal atrophy was found to be seen among 60% of the postmenopausal women.[12] Apart from vaginal atrophy, prolapsed uterus and vaginal shortening may contribute to dyspareunia among menopausal women.[2]
Psychological symptoms of menopause
About 45% of menopausal women experience various psychological issues such as insomnia, irritability, depression, anxiety, poor self-confidence, and difficulty in concentrating on a task.[1] It has been evident that during menopausal transition women tend to experience more sleep disturbances which were confirmed by both actigraphy and self-report.[13,14] About 40–60% of the menopausal women experience insomnia.[15] A study found that 59.8% of postmenopausal women had depression, of which 39.8%, 16%, and 4% had mild, moderate, and severe levels of depression, respectively, as assessed using the Hamilton depression rating scale.[16] Another study found that women tend to experience severe anxiety symptoms during early or late perimenopausal as well as postmenopausal periods when compared to their premenopausal periods with odds ratios of 1.56–1.61.[17] Estrogen modulates the serotonin by enhancing its presynaptic reuptake. Alterations in the levels of female reproductive hormones can impact the neurotransmission in their brain, mainly that of serotonin (5-Hydroxytryptamine/5–HT) and gamma-aminobutyric acid which tend to contribute to the development of psychological symptoms in postmenopausal women.[18]
Paroxetine
Paroxetine is a selective serotonin reuptake inhibitor (SSRI) antidepressant.[19] It exerts its effect by boosting 5-HT neurotransmission in the brain. [Figure 3] summarizes the antidepressant mechanism of action of paroxetine.[20]
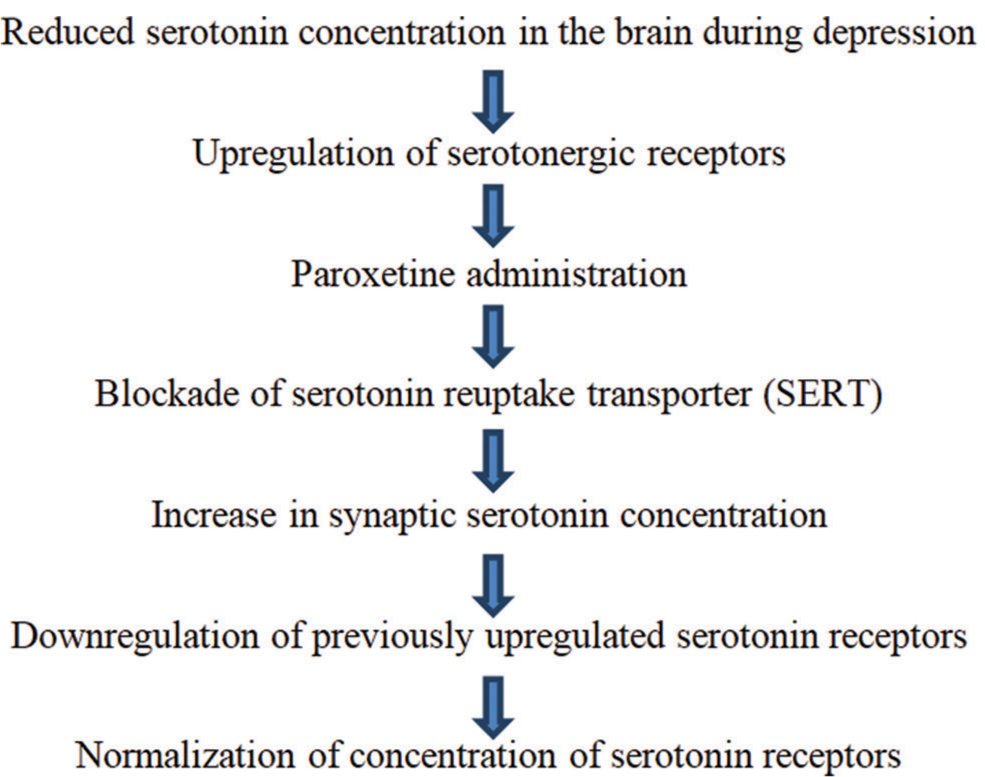
- Mechanism of antidepressant action of Paroxetine.
Paroxetine is also known to have an affinity for various receptors such as serotonergic 5 HT2, dopaminergic D2, muscarinic, adrenergic (α and β), and histaminergic H1 receptors, which contributes to its antidepressant as well as adverse effect profiles.[20,21] Paroxetine was previously accepted by the United States Food and Drug Administration (U.S. FDA) for treating panic disorder, major depressive disorder, post-traumatic stress disorder, obsessive-compulsive disorder, social phobia, generalized anxiety disorder, and premenstrual dysphoric disorder.[19]
Paroxetine for managing the hot flashes of menopause
In 2013, paroxetine mesylate got approval from the U.S. FDA regarding treating moderate and severe hot flashes, thus presenting it as the very first non-hormonal management option for climacteric symptoms of menopause.[22] Paroxetine controlled release (CR) can be used for menopausal vasomotor symptom management within a dose range of 12.5–25 mg.[20,23] [Figure 4] below summarizes the mechanism of paroxetine’s action in the management of hot flushes/flashes of menopause.[22,24]
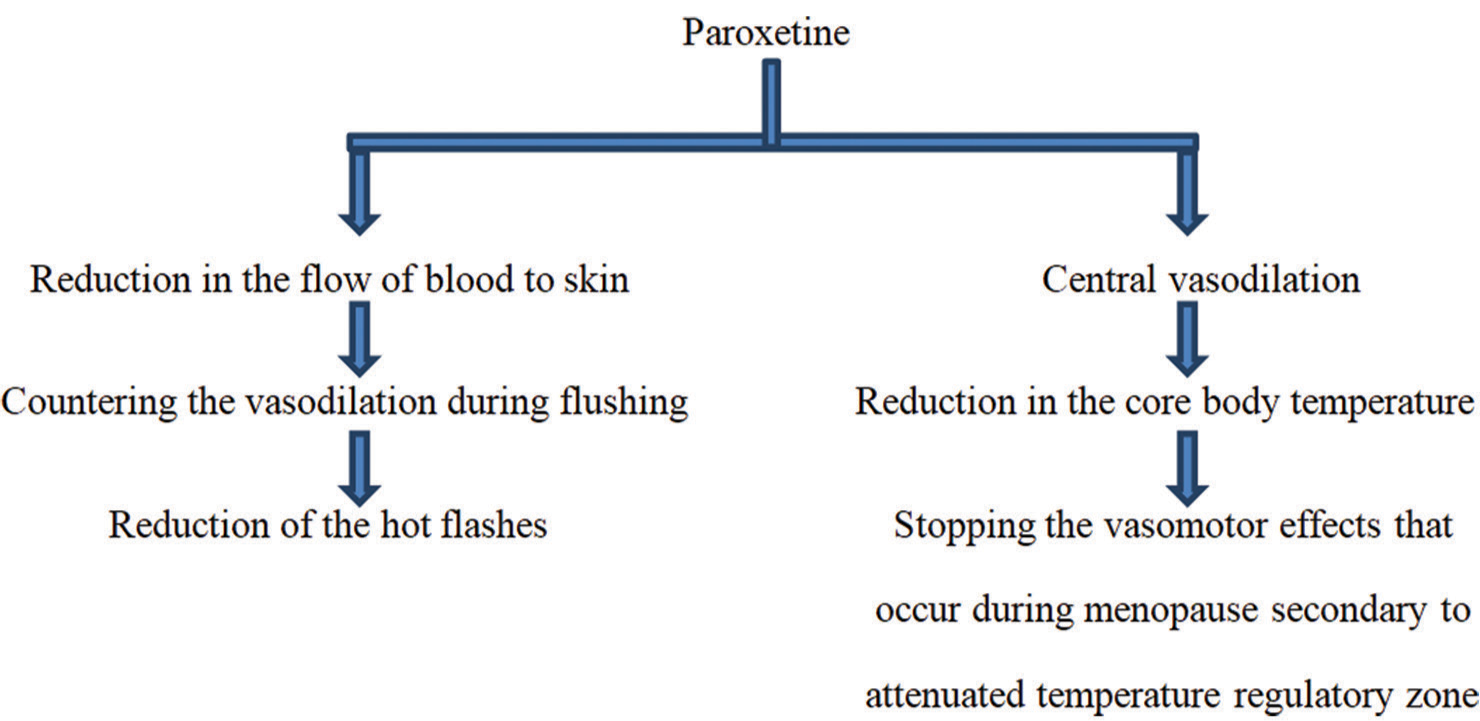
- Mechanism of paroxetine’s action regarding the management of menopausal hot flashes.
Rationale for the present study
Literature shows that the reduction of amounts of hormones such as progesterone and estrogen is the main culprit behind the development of symptoms and signs of menopause including the vasomotor symptoms.[24-26] Hormone therapy with estrogen for vasomotor symptoms of menopause was recommended by the American College of Obstetricians and Gynecologists as well as by the American Association of Clinical Endocrinologists as it was found to be an effective first-line option.[25,26] However, hormone therapy for vasomotor symptoms is not concern-free and there may occur numerous adverse effects of hormone therapy. Menopausal hormone therapy (MHT) is associated with various side effects such as breast soreness, vaginal bleeding (with cyclic progestinestrogen therapy), bloating and mood symptoms (mainly with progestin hormonal therapy), and withdrawal bleeding monthly (with cyclic progestin therapy).[27] Such MHT-related side effects can be bothersome for many menopausal women who have already suffered from menopausal symptoms such as hot flushes or flashes, other vasomotor symptoms, psychological symptoms, and urogenital symptoms. Available literature has shown that there was a decline in the MHT prescription rate after the year 2002 from 22.4% (in 1999– 2000) to 11.9% (in 2003–2004).[27,28] Another study found that prescription of MHT was on the rise between 1996 and 2001, it reached a peak in 2001, and then reduced over the year 2003 to 2008 by about 64–67%.[29] Hence, in the background of evidence of declining use of MHT among females during the phase of menopause, there is a need to explore non-hormonal therapy options for better outcomes in managing women suffering from vasomotor symptoms of menopause. Recently, paroxetine mesylate was approved by the U.S. FDA as the first non-hormonal method in the management of vasomotor symptoms of menopause.[22]
Purpose of the present study
This study was conducted with the purpose of helping the postgraduate residents and practicing clinicians including psychiatrists and gynecologists to understand paroxetine’s role in managing bothersome hot flashes of menopause for betterment in patient care and treatment outcomes.
Objective of the present study
This study was conducted with the objective to explore the potential use of paroxetine in managing hot flashes which are one of the vasomotor symptoms of menopause.
MATERIAL AND METHODS
Inclusion criteria
Inclusion norms used in this study included (a) studies that focused on menopausal women both including normal physiological menopause as well as surgical menopause, (b) studies that included the women who fulfilled the criteria of menopause, that is, absence of menses for a consecutive period of 12 months, (c) studies that primarily focused on the use of paroxetine for management of menopausal hot flashes, and (d) those published in English.
Sources of information
For the present study, a search of the literature was carried out online from various useful databases such as MEDLINE, SCOPUS, EMBASE, Web of Science, PubMed, and Google Scholar.
Strategy for searching the literature
The literature search for the present study was limited to previously published reviews, original, meta-analysis, and randomized controlled trial (RCT) studies that were published in the English language from 2000 to 2022. Other types of study such as editorials, letters to editors, abstracts, perspectives, and commentaries were not included. For the purpose of search strategy, literature exploration and the review were carried out by utilizing keywords such as “menopause,” “vasomotor symptoms,” “hot flashes,” “hot flushes,” and “paroxetine.” Variations of terms during the literature search included [“menopause” OR “climacteric” OR “perimenopause” OR “postmenopause”] AND [“paroxetine”] AND [“hot flashes” OR “hot flushes” OR “vasomotor symptoms” OR “climacteric symptoms”]. The period of the literature search was from August 2022 to April 2023.
Process of selecting the records for data collection
In this study, a 4-phase process of preferred reporting items for systematic reviews and meta-analyses statement (PRISMA) was applied.[30] An inceptive literature search using the above-mentioned keywords resulted in 489 articles. After this first phase of “Identification,” 489 articles were processed for the next phase. During the second phase of “Screening”, on the basis of details from titles and abstracts (N=451) as well as removal of duplicates (N=0), 451 articles were excluded as they were completely out of scope for the present study. Hence, after the second phase, a total of 38 articles remained. During the third phase of “Eligibility,” full texts of 38 articles were reviewed and 23 articles were ruled out as they were not in line with the selection criteria of the present study. Hence, after the third phase, a total of 15 articles were left. During the last phase of “Inclusion,” we reviewed 15 articles which were finally incorporated into the present study because they were in line with the selection criteria of the present study.[22,31-44] Particulars of the above phases are emphasized in [Figure 5] below as per the PRISMA guideline.
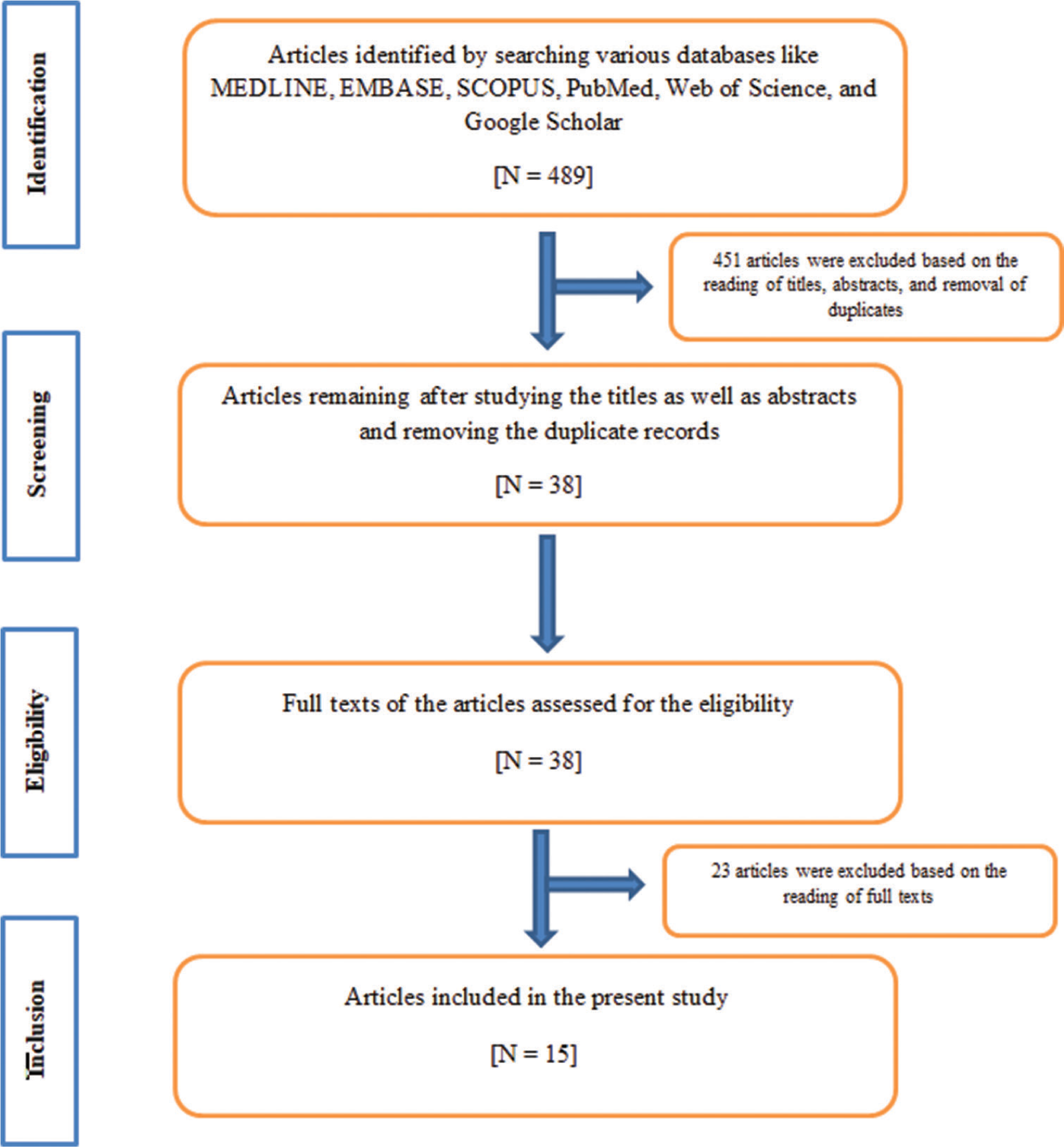
- PRISMA flowchart of the literature selection and data collection strategies for the present study.
RESULTS
Evidence of the therapeutic role of paroxetine in the treatment of menopausal hot flashes
Carroll et al. carried out a systematic review study that consisted of prospective RCTs, retrospective and prospective cohort studies, and case–control trials published in the English language focusing on the primary outcome of vasomotor symptoms of menopause mainly hot flashes and studies on paroxetine versus active or placebo treatment.[22] Data were derived from 194 women with vasomotor symptoms with a history of breast cancer and 1407 women with vasomotor symptoms but without a history of breast cancer. They found that treatment with paroxetine (mesylate and hydrochloride) showed a remarkable decline of hot flash frequency by 33– 67% compared to only 13.7–37.8% decline with placebo treatment of females with menopause irrespective of the history of breast cancer over the period of 6–12 weeks.[22] Treatment of menopausal women with paroxetine for the vasomotor symptoms is safer and effective in a lower-dose range of 7.5–12.5 mg/day. Hence, paroxetine can be regarded as a mainstay treatment modality for menopausal women for whom MHT is either intolerable or inappropriate.[22]
Riemma et al. conducted a systematic review and meta-analysis study consisting of four RCTs conducted on 1482 women with physiological or surgical menopause experiencing hot flushes and insomnia that were randomized to either placebo or lower strength paroxetine.[31] They found that episodes of hot flashes significantly declined among females on paroxetine in contrast to females on placebo treatment.[31] Paroxetine in lower doses can be considered as an efficacious option in managing the menopausal vasomotor symptoms, mainly hot flashes. Paroxetine-treated postmenopausal women showed marked attenuation of hot flush frequency compared to placebo with mean weekly attenuation with mean difference (MD) – 7.97 episodes/week. The hot flush reduction was – 7.89 MD and – 7.63 MD among women having physiological and surgical menopause, respectively. Evidence quality regarding the role of paroxetine in managing vasomotor symptoms was good.[31]
Wei et al. conducted a systematic review and meta-analysis study consisting of RCTs on assessing paroxetine’s effect compared to no treatment or placebo among postmenopausal as well as perimenopausal women with moderate and severe levels of vasomotor symptoms.[32] A total of 2717 perimenopausal and postmenopausal women from nine RCTs were assessed for the efficacy of paroxetine with regard to the frequency of hot flashes. A total of 2667 perimenopausal and postmenopausal women from eight RCTs were assessed for the efficacy of paroxetine with regard to the severity of hot flashes. They found that the treatment of vasomotor symptoms with paroxetine showed a marked mean decline in hot flash frequency by MD of 8.82/week in the 4th week, 8.86/week in the 6th week, and 7.27/week in the 12th week.[32] The same study also showed a marked mean decline in hot flash severity score by MD 3.18 and 2.4/week at the 4th and 12th weeks, respectively. Paroxetine use was linked with side effects such as dizziness and nausea. Evidence quality regarding the role of paroxetine in managing vasomotor symptoms was found to be moderate.[32]
Simon et al. conducted RCT based on 2003 FDA guidance regarding the clinical assessment of MHT in vasomotor symptoms of menopause.[33] A total of 591 and 593 postmenopausal females were randomly allocated to paroxetine 7.5 mg and placebo treatment groups, respectively. In the 12-week study, mean weekly attenuations of vasomotor symptom (VMS) frequencies were –33.0 versus –23.5 at week 4 and were –43.5 versus –37.3 at week 12 on paroxetine 7.5 mg versus placebo, respectively. In the 24-week study, mean weekly attenuations of frequencies of VMS were –28.9 versus –19.0 at week 4 and were –37.2 versus –27.6 at week 12 on paroxetine 7.5 mg versus placebo, respectively. Regarding VMS severity, in the 12-week study, the mean weekly attenuations were –0.09 versus –0.05 at week 4 and were –0.10 and –0.09 at week 12 on paroxetine 7.5 mg versus placebo, respectively. In the 24-week study, mean weekly attenuations in the severity of VMS were –0.09 versus –0.06 at week 4 and were –0.12 versus –0.07 at week 12 on paroxetine 7.5 mg versus placebo, respectively. Hence, they found that paroxetine in the lower dose of 7.5 mg/day lowered the mean weekly frequency as well as the severity of vasomotor symptoms of menopause in comparison to the placebo on weeks 4 and 12 in 24 weeks of treatment. The majority of the treatment-emergent side effects were mild to moderate in intensity, with no notable alterations found in the vitals and laboratory parameters of the study participants. There occurred no paroxetine discontinuation symptoms after stopping the treatment.[33] The discontinuation emergent signs and symptoms (DESS) scale was applied within 7 ± 3 days of the final paroxetine dose. DESS did not show any significant difference between the placebo arm and low dose 7.5 mg paroxetine arm which means that paroxetine did not cause any increased risk of discontinuation symptoms after its stoppage. Low-dose paroxetine was well tolerated by the menopausal women with noteworthy attenuation of frequency as well as severity of menopausal vasomotor symptoms. The beneficial outcome of the paroxetine treatment was persistent over 24-week period of the study.[33]
Capriglione et al. conducted an RCT consisting of 86 women with surgical menopause due to gynecological cancer who had postmenopausal hot flashes and sleep disturbance.[34] Out of the total of 86 women, 80 had completed the study intervention. The mean age of study subjects was 53 years with a range of 36–75 years. Of those 80 participants, 42 and 38 belonged to the 7.5 mg paroxetine and placebo groups, respectively. They found a statistically significant decline in the frequency as well as the severity of vasomotor symptoms of menopause on lower strength of paroxetine (7.5 mg) compared to the placebo on week 4 and week 16. Mean weekly attenuations of VMS frequencies were –31.0 versus –21.5 at week 4 and were –46.5 versus –39.3 at week 16 on 7.5mg paroxetine and placebo treatments, respectively. As regards VMS severity, the mean weekly attenuations were –0.09 versus –0.05 at week 4 on 7.5 mg paroxetine versus placebo treatment, respectively. Statistically significant attenuation of vasomotor symptoms related to night-sleep awakening and improved sleep duration were also seen among the study participants who received paroxetine than those who received placebo. Treatment with paroxetine was beneficial in improving the sleep duration and reducing the severity as well as frequency of hot flashes. Paroxetine was well tolerated and the level of compliance was high.[34]
Stearns et al. conducted RCT consisting of women with physiologic menopause who experienced at the minimum 2–3 episodes of troublesome hot flashes per day or no <14 troublesome hot flashes per week, and those who have quit MHT for a period of at least 6 weeks.[35] Of the total of 165 menopausal women, 56 randomly received placebo treatment while 51 received 12.5 mg/day of CR paroxetine and 58 received 25 mg/day of paroxetine CR. Randomization was done on the ratio of 1:1:1 for a period of 6 weeks. By the 6th week of treatment, the mean frequency of the daily hot flashes was turned down from 7.1 to 3.8 (mean reduction of 3.3.) in women who received 12.5 mg/day of paroxetine CR, from 6.4 to 3.2 (mean reduction of 3.2) in women who received 25 mg/day paroxetine CR, and from 6.6 to 4.8 (mean reduction of 1.8) in those who received placebo treatment for hot flashes. At the end of 6 weeks of management, the median reduction in hot flash composite scores was 64.6%, 62.2%, and 37.8% among menopausal women who received 25 mg/day of paroxetine CR, 12.5 mg/day of paroxetine CR, and placebo treatment, respectively.[35] CR formulation of paroxetine can be effectively used as a non-hormonal treatment alternative to MHT for managing postmenopausal hot flashes.
Soares et al. conducted a controlled clinical trial in which 64 women in perimenopausal as well as postmenopausal phases without anxiety and depression who were experiencing vasomotor symptoms were included in the study.[36] Fifty women completed the study. At the entry point of the study, women who had 17 hot flashes every week received MHT over a period of >5 years and ceased the treatment for <1 year before the onset of the study. Treatment with paroxetine CR in a dose range of 12.5–25 mg/day was found to be more efficacious compared to placebo in controlling the hot flashes of menopause. The mean reduction in hot flashes was 6.1 versus 2.8 per week with paroxetine CR and placebo, respectively, at P = 0.03.[36] Paroxetine CR can be effectively used as an alternative treatment option in treating postmenopausal as well as perimenopausal vasomotor symptoms among females who have discontinued the MHT.
Stearns et al. conducted a prospective RCT study on 151 women who experienced not <2 bothersome hot flashes every day or 14 bothersome hot flashes every week over a period of 1 month or longer.[37] They found that hot flash frequency was minimized by 40.6% by paroxetine 10 mg/day compared to 13.7% by placebo (P = 0.0006). Hot flash frequency was minimized by 51.7% by paroxetine 20 mg/day compared to 26.6% by placebo (P = 0.002). The hot flash composite score was reduced by 45.6% by 10 mg paroxetine compared to 13.7% by placebo (P = 0.0008). On the other hand, 20 mg paroxetine administration was associated with a reduction of hot flash composite score by 56.1% compared to 28.8% by placebo (P = 0.004).[37] The efficacy of both doses of paroxetine was found to be similar, but treatment discontinuation was lesser at lower doses. There was a significant improvement in sleep with paroxetine 10 mg/day compared to placebo (P = 0.01).[37] Paroxetine can be effectively used as a treatment option for hot flashes of premenopausal, perimenopausal, and postmenopausal women with or without earlier history of breast cancer.[37]
David et al. conducted a clinical review study on menopausal women experiencing bothersome vasomotor symptoms and observed that paroxetine in a lower dose was effectively used as non-hormonal therapy in managing menopausal vasomotor symptoms.[38] The recommended daily dose of paroxetine is 7.5 mg at bedtime. Treatment with paroxetine was associated with a significant decline in severity as well as frequency of hot flashes. Paroxetine can be used for menopausal females for the management of vasomotor symptoms where MHT is either not preferred or contraindicated. Level I evidence has been shown in the case of treatment of vasomotor symptoms with paroxetine. Both the mesylate and hydrochloride salts of paroxetine have shown efficacy, but paroxetine hydrochloride can be used when there exist issues such as cost and availability.[38] Dose-dependent side effects of paroxetine are dizziness, fatigue, and nausea.[38] As paroxetine is a strong cytochrome P450 2D6 isoenzyme (CYP2D6) inhibitor, its utilization must be avoided among females suffering from carcinoma of the breast who are on tamoxifen because there are more chances of recurrence of carcinoma of the breast with a marked increase in the likelihood of death.[38] In such populations, other safer antidepressants such as desvenlafaxine, venlafaxine, citalopram, or escitalopram can be used, of which desvenlafaxine and venlafaxine have no CYP2D6 inhibiting property. On the other hand, escitalopram and citalopram have weak CYP2D6 inhibiting properties.[38]
Simon et al. conducted an RCT on 42 menopausal women aged 40 years or more with 5–50 hot flashes per week.[39] Those 42 women were randomized to 3:3:1–60 mg raloxifene, placebo, and 20 mg paroxetine over a period of 12 weeks. Diaries related to the severity and frequencies of hot flashes were appraised at intervals of 1 week. After 12 weeks, frequencies of the hot flashes showed mean percent changes of – 14.2%, – 37.4%, and – 49.8% with raloxifene, placebo, and paroxetine, respectively. After 12 weeks, the severity of hot flashes showed mean percent changes of – 9.6%, – 39.9%, and – 36.6% with raloxifene, placebo, and paroxetine, respectively.[39] Hot flash frequencies were lower among the paroxetine group in contrast to the raloxifene group (−49.8% versus −14.2%). The hot flash diary showed reliable trends toward the expected differences between paroxetine and raloxifene groups, indicating that a hot flash diary can be very handy for assessing attenuation in hot flash severity as well as frequencies among postmenopausal females.[39]
Naz et al. conducted an open-label control clinical trial on 180 outpatients with postmenopausal hot flashes.[40] They divided study participants into three groups receiving 12.5 mg paroxetine, 20 mg paroxetine, and placebo. Greene climacteric score (GCS) scaling was used in those three groups to assess the effects of placebo, 12.5 mg paroxetine, and 20 mg paroxetine on the frequency of hot flashes among menopausal females. With regard to baseline, mean GCS scoring frequencies were 2.64 ± 0.29, 2.76 ± 0.23, and 2.76 ± 0.24 among 12.5 mg paroxetine, 20 mg paroxetine, and placebo groups, respectively. At 12 weeks, the mean GCS scoring frequencies were 1.97 ± 0.31, 2.04 ± 0.12, and 2.80 ± 0.24 among 12.5 mg paroxetine, 20 mg paroxetine, and placebo groups, respectively.[40] The frequency of hot flashes with both 12.5 mg and 20 mg paroxetine doses was significantly reduced when compared with placebo among the postmenopausal women.
Portman et al. carried out RCT in which females in a postmenopausal phase aged 40 years and more with seven to eight hot flushes every day or 50–60 hot flushes every week of moderate to severe intensities over the period of 1 month were included in the study by randomly assigning them to 7.5 mg paroxetine and placebo groups for 12 or 24 weeks period.[41] A total of 1184 study subjects were included of which 570 belonged to 24 weeks study and 614 belonged to 12 weeks study. They pooled the data from the 1st to 12th weeks of both 12 and 24 week’s studies. Women who were “hot flash responders” among the paroxetine 7.5 mg group (i.e., those who showed 50% or more attenuation in hot flash frequency from baseline) showed marked improvement in Arizona Sexual Experiences Scale scores at weeks 4, 12, and 24 than women who were hot flash non-responders. No statistically significant alterations from baseline sexual functioning or weight were recorded in the 7.5 mg paroxetine group. Rather, a small significant increase in parameters such as body mass index and body weight was seen among women from the placebo group at week 4 of the treatment.[41] Paroxetine with a least strength of 7.5 mg/day is a potent non-hormonal treatment of choice in managing the menopausal vasomotor symptoms of moderate and severe intensities. Such a low-dose paroxetine formulation is not associated with any significant alteration in sexual functioning and body weight over a treatment period of 24 weeks.[41]
Pinkerton et al. carried out RCT in which menopausal women who had >7 or 8 hot flashes every day, or 50–60 hot flashes every week of moderate to severe intensities over the period of at least 30 days or more before the entry point of the study were incorporated.[42] Postmenopausal women with vasomotor symptoms of moderate and severe grades were randomly allocated placebo (n=593) and paroxetine 7.5 mg (n=591) groups. At the onset of the study, participants experienced 3.6 awakenings per night due to the vasomotor symptoms of menopause. Such night-time sleep fragmentations due to vasomotor symptoms were found to be greatly decreased within 4 weeks of onset of treatment with 7.5 mg paroxetine (39% reduction with paroxetine vs. 28% reduction with placebo at P = 0.0049). Attenuation in night-time awakening was persistent throughout a period of 12–24 weeks of management with paroxetine. Furthermore, pharmacotherapy with 7.5 mg paroxetine significantly improved the total sleep duration during night-time (at week 4, total sleep duration was increased by +31 min with paroxetine versus +16 min with placebo at P = 0.0075).[42] The use of lower-strength paroxetine (7.5 mg) in managing the vasomotor symptoms of menopause increases total nighttime sleep duration as well as significantly reduces night-time awakenings that occur secondary to vasomotor symptoms.[42]
Stearns et al. conducted a pilot trial in which 30 breast cancer survivor females (three premenopausal and 27 postmenopausal) who had a minimum of two hot flashes every day or a minimum of 14 hot flashes every week over a minimum 1 month period were selected for the participation in the study to assess the effectiveness of paroxetine on frequency and severity related with hot flashes.[43] Twenty-seven females completed the 6 week long study. Those women were asked to complete hot flash diaries throughout the study on a daily basis. For the initial 1 week period, women did not receive paroxetine, for another 1 week, they received 10 mg paroxetine/day, and for another 4 weeks period, they received 20 mg paroxetine/ day. Mean reductions in the severity and the frequency of hot flashes were 75% and 67%, respectively.[43] The study also showed a significant improvement in other parameters such as sleep, anxiety, quality of life, and depression among the study participants.[43] Twenty-five (83%) participants continued taking paroxetine until the end of the study. Two women discontinued and the other two women had dose reduction due to somnolence caused by paroxetine. One woman discontinued paroxetine due to perceived anxiety while on treatment.[43] Paroxetine hydrochloride is an effective treatment option in treating hot flashes among pre-and postmenopausal women who are survivors of carcinoma breast.[43]
Weitzner et al. conducted a pilot trial to assess the safety and effectiveness of paroxetine in the management of hot flashes and disturbances of sleep among females with carcinoma of the breast.[44] A total of 13 females took part in the study. The mean age of study subjects was 52 years with a range of 43– 60 years. Of those 13, 3, and 10 women were perimenopausal and postmenopausal, respectively. All the study subjects were suffering from hot flashes. After treatment with 20 mg/ day paroxetine, the mean rating of severity of hot flashes notably minimized from “quite a bit” or “extremely severe” to “moderately severe” (mean of 2.08 and P = 0.002). There was a reduction of percentage from 100 to 38 regarding the subjects who marked the rating of hot flashes as “extremely severe” or “quite a bit” (P = 0.008). After paroxetine therapy, the quality of sleep also showed noteworthy improvement from a rating of “fairly bad” to “very good” (mean of 1.85 vs. 0.77).[44] Evidence of quality regarding treatment with paroxetine for vasomotor symptoms was Level I.[45] Table 1 above summarizes various findings from all the 15 studies included in this review in a concise manner.
| Author, Year and Reference number | Type of study | Sample size | Dose of Paroxetine | Frequency of Hot flashes | Severity of Hot flashes | ||
|---|---|---|---|---|---|---|---|
| Placebo treated group | Paroxetine treated group |
Placebo treated group | Paroxetine treated group |
||||
| Carroll et al., 2015,[22] | Systematic review | 194 women with breast cancer and 1407 women without breast cancer who had menopausal VMS | 7.5 mg to 12.5 mg/day | Declined by 13.7 to 37.8% | Declined by 33 to 67% | ________ | Significant decline in severity of hot flashes mainly at week 4 than week 12 |
| Riemma et al., 2019,[31] | Systematic review and meta-analysis | 1482 women with physiological or surgical menopause who experience hot flashes and insomnia | 7.5 mg to 12.5 mg/day | Declined by -5.42 episodes/ week | Declined by -10.51 episodes/ week (with a mean difference of -7.97 (-10.51, -5.42) episodes/ week) |
________ | _________ |
| Wei et al., 2016,[32] | Systematic review and meta-analysis | 2717 and 2667 perimenopausal and postmenopausal women were evaluated for efficacy of paroxetine for frequency and severity of hot flashes | 7.5 mg to 25 mg/day |
0 | Mean decline was by MD of 8.82/week, 8.86/week, 7.27/week in 4th, 6th, and 12thweeks respectively | 0 | Mean decline was by MD of 3.18/week and 2.4/week at 4thand 12thweeks respectively |
| Simon et al., 2013,[33] | RCT | 591 and 593 postmenopausal women were randomly assigned paroxetine 7.5 mg and placebo groups respectively | 7.5 mg/day | Mean weekly reduction were -23.5 at 4thweek and -37.3 at 12thweek in placebo arm of the 12-week study. Mean weekly reduction were -19.0 and -27.6 at 4thand 12thweeks respectively in placebo arm of the 24-week study. |
Mean weekly reduction were -33.0 at 4thweek and -43.5 at 12thweek in paroxetine 7.5 mg arm of the 12-week study. Mean weekly reduction were -28.9 and -37.2 at 4thand 12thweeks respectively in paroxetine 7.5 mg arm of the 24-week study. |
Mean weekly reductions were -0.05 at 4thweek and -0.09 at 12thweek in placebo arm of the 12-week study. Mean weekly reduction were -0.06 and -0.07 at 4thand 12thweeks respectively in placebo arm of the 24-week study. |
Mean weekly reductions were -0.09 at 4thweek and -0.10 at 12thweek in paroxetine 7.5 mg arm of the 12-week study. Mean weekly reduction were -0.09 and -0.12 at 4thand 12thweeks respectively in paroxetine 7.5 mg arm of the 24-week study. |
| Capriglione et al., 2016,[34] |
RCT | Out of 86, 80 women with surgical menopause completed the study. 42 were in Paroxetine group and 38 were in placebo group | 7.5 mg/day | Mean weekly reductions were -21.5 and -39.3 at weeks 4thand 16threspectively. | Mean weekly reduction were -31.0 and -46.5 at weeks 4thand 16threspectively. | Mean weekly reduction was -0.05 at week 4. | Mean weekly reduction was -0.09 at week 4. |
| Stearns et al., 2003[35] | RCT | 165 menopausal women were included of which 56, 51, and 58 received placebo, 12.5 mg, and 25 mg paroxetine CR | 12.5 mg/day and 25 mg/day paroxetine CR | Mean reduction of 1.8. | Mean reductions were 3.3 and 3.2 on paroxetine CR 12.5 mg and 25 mg doses respectively. | Median reduction was 37.8%. | Median reductions were 62.2% and 64.6% on paroxetine CR 12.5 mg and paroxetine CR 25 mg respectively. |
| Soares et al., 2008,[36] | Controlled clinical trial | 50 out of 64 perimenopausal and postmenopausal women completed the study. | 12.5 mg to 25 mg/day | Mean reduction of 2.8 hot flashes/week. | Mean reduction of 6.1 hot flashes/week. | No significant differences were observed at the baseline between paroxetine and placebo groups as regards to the severity of VMS. | |
| Stearns et al., 2005,[37] | Prospective RCT | 151 women with at least 2 to 14 hot flashes per week over 1 or more months. | 10 mg/day and 20 mg/day | Declined by 13.7%. Declined by 26.6%. |
Declined by 40.6% on 10 mg/day of paroxetine. Declined by 51.7% by 20 mg/day of paroxetine. |
Composite score was declined by 13.7%. Composite score was declined by 28.8%. |
Composite score was declined by 45.6% on 10 mg paroxetine. Composite score was declined by 56.1% on 20 mg paroxetine. |
| David et al., 2022,[38] |
Clinical review | Total 1795 premenopausal, perimenopausal and postmenopausal women from four studies | 7.5 mg/day | __________ | Significant decline compared to placebo. | __________ | Significant decline compared to placebo. |
| Simon et al., 2014,[39] | RCT | 42 menopausal women, randomized on basis of 3:3:1 to 60 mg raloxifene, placebo, and paroxetine respectively | 20 mg/day | Mean percent declines of -37.4% on placebo and -14.2% on raloxifene. | Mean percent decline of -49.8% on paroxetine. | Mean percent declines of -39.9% on placebo and -9.6% on raloxifene. | Mean percent decline of -36.6% on paroxetine. |
| Naz et al., 2019,[40] |
Open label control clinical trial | 180 postmenopausal women with hot flashes | 12.5 mg/day and 20 mg/day paroxetine |
Baseline mean GCS frequency on placebo: 2.76 ± 0.24. At 12 weeks, mean GCS frequency on placebo: 2.80 ± 0.24. |
Baseline mean GCS frequencies on paroxetine 12.5 mg and 20 mg: 2.64 ± 0.29 and 2.76 ± 0.23 respectively. At 12 weeks, mean GCS frequencies on paroxetine 12.5 mg and 20 mg: 1.97 ± 0.31 and 2.04 ± 0.12 respectively. |
__________ | __________ |
| Portman et al., 2014,[41] | RCT | 1184 postmenopausal women with hot flashes | 7.5 mg/day | At 4thweek, placebo treated women showed little significant increase in body weight and BMI. | Hot flash responder women with more than 50% reduction of hot flash frequency showed notably enhanced ASEX scores at 4th, 12th, and 24thweeks. There were no statistically significant changes in body weight and sexual functioning on 7.5 mg paroxetine. | __________ | __________ |
| Pinkerton et al., 2015,[42] |
RCT | 591 and 593 postmenopausal women with hot flashes were randomized to paroxetine 7.5 mg and placebo groups respectively | 7.5 mg/day | Night time sleep fragmentations secondary to VMS were reduced by 39% and 28% on paroxetine 7.5 mg and placebo respectively. Total night time sleep duration was improved by +31 minutes and +16 minutes on paroxetine 7.5 mg and placebo respectively. |
|||
| Stearns et al., 2000,[43] | Pilot trial | 30 (3 premenopausal and 27 postmenopausal) carcinoma breast survivor women with hot flashes. 27 women completed the study. | 10 mg/day during 2ndweek and 20 mg/day during 3rdto 6thweek | __________ | Mean reduction was 67%. | __________ | Mean reduction was 75%. |
| Weitzner et al., 2002,[44] | Pilot trial | 13 (3 premenopausal and 10 postmenopausal) women with carcinoma breast with hot flashes | 10 mg/day for initial 3 nights followed by 20 mg/day every night | __________ | __________ | __________ | Mean severity rating declined from extremely severe to moderately severe. Percentage of study participants that rated severity of hot flashes as extremely severe reduced from 100 to 38. Prior to paroxetine treatment, hot flash severity score was 3.62 ± 0.51 and it was reduced to 2.08 ± 1.32 after paroxetine treatment. |
MD: Mean difference, VMS: Vasomotor symptom, CR: Controlled release, GCS: Greene Climacteric score, ASEX: Arizona sexual experiences scale, BMI: Body mass index.
DISCUSSION
The present study has reviewed the paroxetine’s role and efficacy in the management of menopausal hot flashes. The present study observed that low-dose paroxetine is a beneficial non-hormonal treatment option for the reduction of the severity as well as frequency of menopausal hot flashes. MHT is considered a gold standard treatment option in managing menopausal vasomotor symptoms including hot flushes/ flashes, but it is accompanied by bothersome side effects. MHT increases the risk of suffering from stroke.[46] One RCT has found that progestin plus estrogen hormonal treatment was accompanied by increased stroke risk by 44% among females in the postmenopausal phase.[47] Progestin plus estrogen hormone therapy can cause early adverse effects such as nausea, tenderness in breasts, abdominal bloating, perceived weight gain, and atypical uterine bleeding.[48] It can also cause long-term adverse effects such as stroke, thromboembolism, gallbladder disease, and cancers.[48] A study assessed the cancer risk among MHT users using standardized incidence ratios (SIRs). The same study found that SIRs of any cancer were 1.14, 1.09, and 1.04 following estrogen plus progestin MHT, all MHT, and estrogen-only MHT, respectively. The same study found that the likelihood of endometrial, ovarian, and invasive breast cancers was increased for any type of MHT. The same study also found that the probability of invasive carcinoma of the breast was raised with advancing age for users of estrogen and progestin MHT.[49] Another study found that women receiving MHT showed an increased chance of carcinoma of the breast, stroke, coronary disease of the heart, and pulmonary embolism.[50] Hence, there exist concerns regarding the safety of MHT. Therefore, there is a need to explore the non-hormonal management strategy for treating vasomotor symptoms of menopause. Paroxetine has been newly cleared by the U.S. FDA as a non-hormonal management option for menopausal vasomotor symptoms.[22] A systematic review and meta-analysis study found that compared to placebo, paroxetine and other SSRIs remarkably lowered the frequencies as well as the severity of hot flashes of menopause.[50] A lower dose of paroxetine is required in treating menopausal vasomotor symptoms than those used to manage depression and anxiety.[38] Adverse drug reactions of paroxetine are mainly dependent on the dose strength and commonly consist of nausea, dizziness, and fatigue which are less bothersome than those caused by the MHT.[38] According to a recent systematic review, the most commonly seen adverse effects of paroxetine include nausea, sleep disturbance, and headache. Other rare side effects of paroxetine may include muscle spasms, cramps, feelings of restless leg, and twitching of muscles.[51] From the available literature, overall the adverse effects of paroxetine can be better tolerated and are less bothersome when compared to the adverse outcomes associated with MHT. Among the various antidepressants used in managing menopausal vasomotor symptoms, only paroxetine has level I evidence, while others such as citalopram, escitalopram, and venlafaxine have level II evidence.[45] Unlike several side effects and contraindications for MHT, contraindications for the use of SSRIs (which includes paroxetine) and the serotonin and norepinephrine reuptake inhibitors are very few which include previous history of neuroleptic malignant syndrome, concurrent administration of monoamine oxidase inhibitors, and serotonin syndrome.[45] Sometimes, migrainous headaches can present as one of the menopausal vasomotor symptoms. Paroxetine can be used as a prophylactic measure for managing migraine in menopausal women with vasomotor symptoms.[52] Five studies showed improved night-time sleep among the menopausal women who received low dose paroxetine.[34,37,42-44]
Limitations
A few limitations exist like studies incorporated in the current systematic review did not explore the duration of every vasomotor symptoms and diurnal differences (night vs. daytime) of vasomotor symptoms. Another limitation was that the dose-dependent side effects of paroxetine were not evaluated in detail. Another limitation like relapse of vasomotor symptoms following discontinuation of paroxetine was not evaluated in detail.
CONCLUSION
As MHT is either contraindicated or not preferred by all menopausal women with vasomotor symptoms due to associated bothersome side effects, there is a need to find a non-hormonal treatment option with a tolerable side effect profile. Paroxetine has shown a very good level I evidence in the reduction of intensity as well as frequency of vasomotor symptoms of menopause. Paroxetine in a lower dose is beneficial for managing vasomotor symptoms in women having physiological as well as surgical menopause.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The author(s) confirm that there was no use of Artificial Intelligence (AI)-Assisted Technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Menopause In: StatPearls. Treasure Island, FL: StatPearls Publishing; 2022. Available from: https://www.ncbi.nlm.nih.gov/books/NBK507826 [Last accessed on 2023 Jan 29]
- [Google Scholar]
- Menopausal Symptoms and their Management. Endocrinol Metab Clin North Am. 2015;44:497-515.
- [CrossRef] [PubMed] [Google Scholar]
- Relationships between Menopausal and Mood Symptoms and EEG Sleep Measures in a Multi-Ethnic Sample of Middle-Aged Women: The SWAN Sleep Study. Sleep. 2011;34:1221-32.
- [CrossRef] [PubMed] [Google Scholar]
- Epidemiology of the Symptoms of Menopause-An Intercontinental Review. Prz Menopauzalny. 2014;13:203-11.
- [CrossRef] [PubMed] [Google Scholar]
- Genetics of Menopause-Associated Diseases. Maturitas. 2001;40:103-16.
- [CrossRef] [PubMed] [Google Scholar]
- Menopause. 2022. Geneva: World Health Organization; Available from: https://www.who.int/news-room/fact-sheets/detail/menopause [Last accessed on 2023 Feb 02]
- [Google Scholar]
- Duration of Vasomotor Symptoms in Middle-Aged Women: A Longitudinal Study. Menopause. 2009;16:453-7.
- [CrossRef] [PubMed] [Google Scholar]
- Premenopausal Vasomotor Symptoms in an Ethnically Diverse Population. Menopause. 2014;21:153-8.
- [CrossRef] [PubMed] [Google Scholar]
- Symptoms Associated with Menopausal Transition and Reproductive Hormones in Midlife Women. Obstet Gynecol. 2007;110:230-40.
- [CrossRef] [PubMed] [Google Scholar]
- Management of Hot Flashes in Women with Breast Cancer. Curr Oncol. 2010;17:81-6.
- [CrossRef] [PubMed] [Google Scholar]
- Self-reported Urogenital Symptoms in Postmenopausal Women: Women's Health Initiative. Maturitas. 2004;49:292-303.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence and Impact of Vaginal Symptoms among Postmenopausal Women. J Sex Med. 2009;6:2133-42.
- [CrossRef] [PubMed] [Google Scholar]
- Sleep Disturbance during the Menopausal Transition in a Multi-Ethnic Community Sample of Women. Sleep. 2008;31:979-90.
- [Google Scholar]
- Actigraphy-defined Measures of Sleep and Movement across the Menstrual Cycle in Midlife Menstruating Women: Study of Women's Health Across the Nation Sleep Study. Menopause. 2015;22:66-74.
- [CrossRef] [PubMed] [Google Scholar]
- Sleep and Sleep Disorders in the Menopausal Transition. Sleep Med Clin. 2018;13:443-56.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence of Depression in Postmenopausal Women. Jundishapur J Chronic Dis Care. 2015;4:e27521.
- [CrossRef] [Google Scholar]
- Does Risk for Anxiety Increase during the Menopausal Transition? Study of Women's Health Across the Nation. Menopause. 2013;20:488-95.
- [CrossRef] [PubMed] [Google Scholar]
- Psychological and Social Aspects of Menopause. A Multidisciplinary Look at Menopause. InTech 2017 Available from: 10.5772/intechopen.69078 [Last accessed on 2023 Sep 29]
- [CrossRef] [Google Scholar]
- Paroxetine In: Grandy MM, Muntner N, eds. Stahl's Essential Psychopharmacology Prescriber's Guide (5th ed). New York: Cambridge University Press; 2014. p. :513-6.
- [Google Scholar]
- Paroxetine In: StatPearls. Treasure Island, FL: StatPearls Publishing; 2022. Available from: https://www.ncbi.nlm.nih.gov/books/NBK526022 [Last accessed on 2023 Feb 03]
- [Google Scholar]
- Clinical Pharmacokinetics of Selective Serotonin Reuptake Inhibitors. Clin Pharmacokinet. 1993;24:203-20.
- [CrossRef] [PubMed] [Google Scholar]
- Critical Appraisal of Paroxetine for the Treatment of Vasomotor Symptoms. Int J Womens Health. 2015;7:615-24.
- [CrossRef] [PubMed] [Google Scholar]
- Paroxetine Hydrochloride. Profiles Drug Subst Excip Relat Methodol. 2013;38:367-406.
- [CrossRef] [PubMed] [Google Scholar]
- Menopausal Hot Flushes and Night Sweats: Where are we now? Climacteric. 2011;14:515-28.
- [CrossRef] [PubMed] [Google Scholar]
- American Association of Clinical Endocrinologists Medical Guidelines for Clinical Practice for the diagnosis and treatment of menopause. Endocr Pract. 2011;17(Suppl 6):1-25.
- [CrossRef] [PubMed] [Google Scholar]
- 141: Management of Menopausal Symptoms. Obstet Gynecol. 2014;123:202-16.
- [CrossRef] [PubMed] [Google Scholar]
- UpToDate. Treatment of Menopausal Symptoms with Hormone Therapy. 2022. UpToDate Available from: https://www.uptodate.com/contents/treatment-of-menopausal-symptoms-with-hormone-therapy [Last accessed on 2023 Feb 04]
- [Google Scholar]
- A Sustained Decline in Postmenopausal Hormone Use: Results from the National Health and Nutrition Examination Survey, 1999-2010. Obstet Gynecol. 2012;120:595-603.
- [CrossRef] [PubMed] [Google Scholar]
- Trends in Prescribing Menopausal Hormone Therapy and Bisphosphonates in Australia and Manitoba, Canada and Adherence to Recommendations. J Womens Health (Larchmt). 2020;29:177-86.
- [CrossRef] [PubMed] [Google Scholar]
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009;6:e1000097.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy of Low-Dose Paroxetine for the Treatment of Hot Flushes in Surgical and Physiological Postmenopausal Women: Systematic Review and Meta-Analysis of Randomized Trials. Medicina (Kaunas). 2019;55:554.
- [CrossRef] [PubMed] [Google Scholar]
- Effect and Safety of Paroxetine for Vasomotor Symptoms: Systematic Review and Meta-Analysis. BJOG. 2016;123:1735-43.
- [CrossRef] [PubMed] [Google Scholar]
- Low-dose Paroxetine 7.5 mg for Menopausal Vasomotor Symptoms: Two Randomized Controlled Trials. Menopause. 2013;20:1027-35.
- [CrossRef] [PubMed] [Google Scholar]
- Role of Paroxetine in the Management of Hot Flashes in Gynecological Cancer Survivors: Results of the First Randomized Single-Center Controlled Trial. Gynecol Oncol. 2016;143:584-8.
- [CrossRef] [PubMed] [Google Scholar]
- Paroxetine Controlled Release in the Treatment of Menopausal Hot Flashes: A Randomized Controlled Trial. JAMA. 2003;289:2827-34.
- [CrossRef] [PubMed] [Google Scholar]
- Paroxetine Versus Placebo for Women in Midlife After Hormone Therapy Discontinuation. Am J Med. 2008;121:159-62.e1.
- [CrossRef] [PubMed] [Google Scholar]
- Paroxetine is an Effective Treatment for Hot Flashes: Results from a Prospective Randomized Clinical Trial. J Clin Oncol. 2005;23:6919-30.
- [CrossRef] [PubMed] [Google Scholar]
- A Clinical Review on Paroxetine and Emerging Therapies for the Treatment of Vasomotor Symptoms. Int J Womens Health. 2022;14:353-61.
- [CrossRef] [PubMed] [Google Scholar]
- Diary of Hot Flashes Reported upon Occurrence: Results of a Randomized Double-Blind Study of Raloxifene, Placebo, and Paroxetine. Menopause. 2014;21:938-44.
- [CrossRef] [PubMed] [Google Scholar]
- The Role of Paroxetine in Postmenopausal Hot-Flashes Frequency Reduction. Pak J Med Dent. 2019;8:22-6.
- [Google Scholar]
- Effects of Low-Dose Paroxetine 7.5 mg on Weight and Sexual Function during Treatment of Vasomotor Symptoms Associated with Menopause. Menopause. 2014;21:1082-90.
- [CrossRef] [PubMed] [Google Scholar]
- Low-Dose Paroxetine (7.5 mg) Improves Sleep in Women with Vasomotor Symptoms Associated with Menopause. Menopause. 2015;22:50-8.
- [CrossRef] [PubMed] [Google Scholar]
- A Pilot Trial Assessing the Efficacy of Paroxetine Hydrochloride (Paxil) in Controlling Hot Flashes in Breast Cancer Survivors. Ann Oncol. 2000;11:17-22.
- [CrossRef] [PubMed] [Google Scholar]
- A Pilot Trial of Paroxetine for the Treatment of Hot Flashes and Associated Symptoms in Women with Breast Cancer. J Pain Symptom Manage. 2002;23:337-45.
- [CrossRef] [PubMed] [Google Scholar]
- Nonhormonal Management of Menopause-Associated Vasomotor Symptoms: 2015 Position Statement of the North American Menopause Society. Menopause. 2015;22:1155-74.
- [CrossRef] [PubMed] [Google Scholar]
- Stroke Risk in Women: The Role of Menopause and Hormone Therapy. Lancet Neurol. 2012;11:82-91.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of Estrogen Plus Progestin on Stroke in Postmenopausal Women: The Women's Health Initiative: A Randomized Trial. JAMA. 2003;289:2673-84.
- [CrossRef] [PubMed] [Google Scholar]
- Oral Oestrogen and Combined Oestrogen/Progestogen Therapy Versus Placebo for Hot Flushes. Cochrane Database Syst Rev. 2004;2004:CD002978.
- [CrossRef] [PubMed] [Google Scholar]
- Menopausal Hormone Therapy and Cancer Risk: An Overestimated Risk? Eur J Cancer. 2017;84:60-8.
- [CrossRef] [PubMed] [Google Scholar]
- SSRIs for Hot Flashes: A Systematic Review and Meta-Analysis of Randomized Trials. J Gen Intern Med. 2014;29:204-13.
- [CrossRef] [PubMed] [Google Scholar]
- The Efficacy and Safety of Selective Serotonin Reuptake Inhibitors and SerotoninNorepinephrine Reuptake Inhibitors in the Treatment of Menopausal Hot Flashes: A Systematic Review of Clinical Trials. Iran J Med Sci. 2022;47:173-93.
- [Google Scholar]
- Current Treatment Options: Headache Related to Menopause-Diagnosis and Management. Curr Treat Options Neurol. 2018;20:7.
- [CrossRef] [PubMed] [Google Scholar]







