Translate this page into:
Appraising the Antimicrobial Effect of 2% Grape Seed Extract Mouthwash on Periodontal Pathogens: A Clinicomicrobiological Analysis
*Corresponding author: Prerna Singh, Department of Periodontics, PMNM Dental College and Hospital, Bagalkot - 587103, Karnataka, India. hannahsonpuladas@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Koregol AC, Kalburgi NB, Singh P, Hannahson P, Sulakod K. Appraising the antimicrobial effect of 2% grape seed extract mouthwash on periodontal pathogens: A clinicomicrobiological analysis. Glob J Med Pharm Biomed Update 2023;18:5.
Abstract
Objectives:
The seeds of Vitis vinifera (Grape) are rich in polyphenolic compounds especially proanthocyanidins that show antimicrobial activity as well as have the potential to halt the progression of gingival inflammation by hindering the activity of interstitial collagenase. The aim was to evaluate and compare the effect of Grape seed extract (GSE) and Chlorhexidine mouthwash on Streptoccocus mitis, Streptococcus salivarius, and Aggregatibacter actinomycetemcomitans and correlate with the clinical parameters.
Material and Methods:
In this randomized, controlled, and double-blinded study, 75 participants were selected from the undergraduate section and divided into three groups, Group A: 25; grape seed extract (2%) mouthwash, Group B: 25; chlorhexidine (0.2%) mouthwash, and Group C: 25; placebo mouthwash. Participants were stipulated to use their assigned mouthwash for 7 days. The supragingival plaque was collected in reduced transport fluid at baseline and 7 days post-intervention and sent for cultural analysis of S. mitis, S. salivarius, and A. actinomycetemcomitans. Colony-forming units (CFUs) were counted and compared for individually selected pathogens at 0 and 7 days among the 3 groups. At each visit, participants were also examined for any clinical changes.
Results:
Mean scores of all clinical parameters (P = 0.05) and mean CFU of S. mitis, S. salivarius, and A. actinomycetemcomitans (P < 0.001) in Groups A and B (Test Groups) differed significantly as compared to Group C (Control Group) at 7 days post regimen. Intragroup comparison revealed a significant reduction in the mean scores on the 7th day of mouthwash use as compared to baseline in Groups A and B, while Group C showed no significant difference.
Conclusion:
It was observed that GSE mouthwash has shown a positive effect in reducing selected periodontal pathogens and improvement of clinical parameters when compared to control. It showed comparable efficacy when compared to chlorhexidine. Its biocompatibility, cost effectiveness, easy availability, and no reported topical side effects make it a potential alternative to chlorhexidine. It efficaciously supplements the periodontal therapy.
Keywords
Grape seed extract
Chlorhexidine
Plant-derived antimicrobials
Proanthocyanadins
Periodontal pathogens
INTRODUCTION
Oral, gingival, and periodontal diseases are major contributors to community health problems globally. Their impact on an individual’s well-being is substantial. The WHO report 2012 states that periodontal disease affects 20–50% of the population worldwide. Extreme periodontal annihilation resulting in loss of teeth is seen in 15–20% of adults in the age range of 35–44 years.[1] The main etiological factor for gingivitis and periodontitis is dental plaque biofilm. It is an adherent, polymicrobial colonies within the matrix of exo-polysaccharide, containing an array of diverse species within its ecological alcove.[2] Kumar 2019 states that although mechanical plaque control methods have the potential to maintain adequate levels of oral hygiene, such methods are not being employed accurately.[3] The outand-out removal of dental plaque by mechanical means is difficult even for the most motivated individuals. On the other hand, the impact of its sub-standard control and recolonization; on oral, gingival, and periodontal health cannot be underestimated. This gave way to the advancement of a multitude of chemotherapeutic plaque control agents, among which the gold standard is chlorhexidine.[4,5] Yet, it has its shortcomings which have made its continual use inexpedient such as tooth discoloration, oral mucosal erosion, and bitter taste.[6] Attention has recently been shifted toward antimicrobial compounds procured from plants as an alternative to the existing synthetical, due to their incredible biocompatibility and minimal side effects.[7]
Grape Seed Extract (GSE) of Vitis vinifera (V. Vinifera) is a prospering medical agent, gaining popularity in the field of medicine and dentistry due to the abundance of bioactive phenolic compounds. About 60–70% of the extractable polyphenols reside in the seeds of V. vinifera. The most abundant polyphenolic compounds present in grape seed are proanthocyanidins (PAs) which account for their anti-inflammatory, antioxidant, anti-proliferative, and cytoprotective properties. These flavonoids are natural collagen cross-linking[2] and strong oxygen-scavenging agents.[8] These properties contribute to the potent anti-inflammatory and anti-oxidant effect of GSE and make it a potential candidate as an anti-inflammatory agent to reduce the development and progression of gingival inflammation.
There exists a rhythmic variation in the bacterial composition of plaque. Nonetheless, streptococci species always predominate and pioneer plaque formation, supervened later by the thriving colonies of Gram-negative cocci and bacilli. As microbial adherence to the tooth surface and each other is an essential step in dental plaque formation, agents demonstrating anti-adhesive effects are worth searching for. Polyphenols in GSE show a tendency to bind with enzyme glucosyltransferase (GTF), one of the key enzymes produced by the streptococci species, required for the initial cohesion of bacteria to the tooth, making it a potent anti-adhesive agent.[2] In addition, GSE has been proven to have anti-bacterial activity. Among the various polyphenols, procyanidins are the frontman of the antibacterial activity, having a MIC score of 1.0 mg mL−1 against the Streptococcus mutans. An efficacious antiplaque agent has to act at different strata of plaque formation such as anti-adhesive; restraining the cohesions of bacteria, diminishing the growth of microbial colonies, and showing antibacterial activity. GSE has shown all these desirable properties in various in vitro studies, but its efficiency in a clinical study is yet to be determined. Besides, given that many compounds are rendered inactive when formulated into a mouthwash, we designed a clinicomicrobiological study to analyze the antimicrobial, antiplaque, and anti-inflammatory efficacy of 2% GSE mouthwash on subjects with mild-to-moderate gingivitis. To determine the antimicrobial efficacy of GSE, we selected Streptococcus mitis and Streptococcus salivarius, the initial colonizers of bacterial plaque and Aggregatibacter actinomycetemcomitans, a keystone pathogen of gingival, and periodontal diseases. The study also investigated the clinical effectiveness of mouthwash as an anti-inflammatory and anti-plaque agent. The hypothesis of the study was that 2% GSE mouthwash will be as efficient as 0.2% CHX Chlorhexidine mouthwash in limiting the number of selected microorganisms as well as showing improvement of the tested parameters when compared with control after 7 days of mouthwash regimen.
MATERIALS AND METHODS
Sample size estimation
The sample size was determined with power of the study at 80% and P < 0.05 using a formula.[9] Approximately 25 subjects per group (75 subjects in total) were estimated to complete the trial.
Recruitment of participants
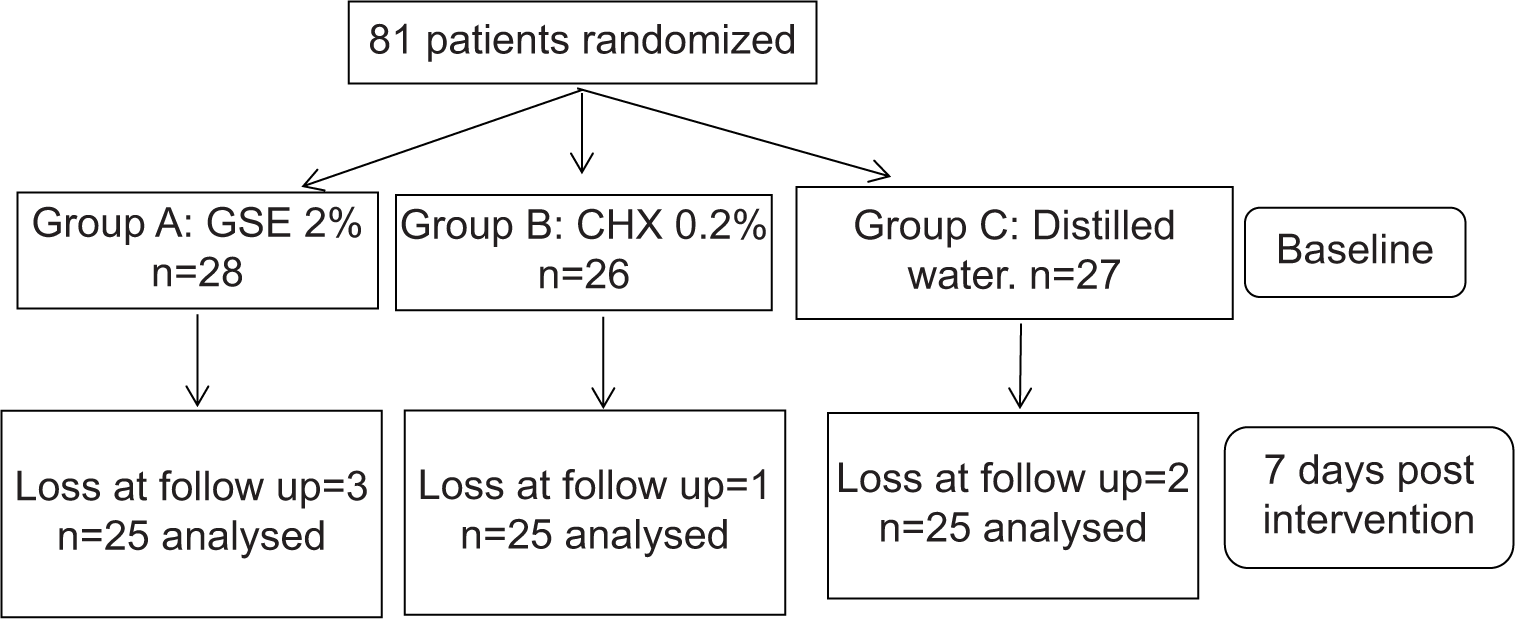
Ethical approval for the execution of the trial was procured by the Institutional Review Board (PMNMDCH/2042/2019-20). After giving an outline of the study to the potential participants, informed consent was signed and acquired from them. Initially, a total of 81 subjects with mild-to-moderate gingivitis of both sexes were recruited in this study, ages ranging from 18 to 30, randomly selected from the undergraduate section. Subjects with systemic diseases that would influence periodontal conditions, subjects who underwent any kind of periodontal treatment in the past 6 months, subjects currently on systemic antibiotics, course of anti-inflammatory, hormonal therapy, corticosteroid therapy, or usage of chemical mouthwash within the previous 3 months, smokers, smokeless tobacco users, pregnant and lactating mothers, and subjects with removable or fixed orthodontic appliances were precluded from the study.
Participants were randomly divided into three groups, Group A received 2% GSE mouthwash, Group B received 0.2% CHX mouthwash, and Group C received distilled water (control group). Nine subjects were lost to follow-up and each group consisted of 25 subjects after 7 days of post-intervention.
For the randomization of participants, the concealed allocation was performed using opaque, amber-colored bottles containing either of the three types of mouthwash. Instructions on how to use the mouthwash were briefed to the participants. The assigned mouthwash had to be used twice a day following brushing and retained for 1 min. Participants were advised not to eat or drink for about half an hour after rinsing with the mouthwash.
In addition, a marketed fluoride dentifrice and a soft bristle brush were dispensed to the participants. They were also demonstrated with the brushing technique.
Clinical data acquisition
Clinical assessment was performed on all the participants at baseline and 7 days post-intervention using the following parameters: Gingival index (GI) (Loe and Silness, 1963), plaque index (PI) (Silness and Loe, 1964), simplified oral hygiene index (OHI-S) (Green JC and Vermillion JR, 1964), and bleeding on probing (Muhlemann and Son, 1971).[10] Participants were asked for any complaints about the taste of the mouthwashes or any sensitivity reaction after its usage on daily basis.
Plaque sampling and microbial analysis
At baseline and 7 days after the use of mouthwash, a sterile curette was used to obtain supragingival plaque. It was collected from the first molars of all four quadrants. Participants were asked to bear any kind of oral hygiene practice for at least 12 h before collection of the sample. The sample collected was stocked in a vial of reduced transport media which was then transported to the laboratory. The processing and analysis of plaque samples was done by a blinded microbiologist.[11] Each of the samples was spread onto mitis-salivarius agar media and anaerobic agar media. For the incubation of the cultures, an anaerobic environment was obtained using 95% nitrogen, 10% hydrogen, and 5% carbon dioxide at 35–37°C.
Preparation of GSE mouthwash
The GSE in the form of powder was obtained from HealthyHey nutraceutical company (Mumbai, India). GSE was manufactured in the GMP facility (Mumbai, India). It contains 95% (w/v) polyphenols, mainly proanthocyanidins (polyphenolic contents determined by the supplier).
2% GSE mouthwash was prepared (2000 microgram/mL).[12] 200 mg of extract was taken in a sterile beaker; it was mixed with 100 mL of distilled water. The beaker containing this mix was placed on a hot plate magnetic stirrer at 60°C. A homogenous solution was obtained. Now to this solution, 900 mL of distilled water was further added to obtain a final volume of 1000 mL. The solution was, then, transferred to an amber colored bottle which was refrigerated for further use.
Statistical analysis
Statistical analysis was done using the software Statistical Package for the Social Sciences, Windows version 22.0, 2013. Intergroup comparison of the mean values was done using a one-way analysis of variance test. For the intergroup comparison of the mean colony-forming units (CFUs) of different microorganisms, Kruskal–Wallis test followed by Mann–Whitney post hoc test was used. For the intragroup comparison of clinical parameters between baseline and 7 days post-intervention, a Student pair t-test was used, while for the mean CFUs of different organisms between baseline and 7 days post intervention period in each study group Wilcoxon Signed-Rank test was used. P < 0.05 delineates the level of significance.
RESULTS
Clinical parameters
Intergroup comparison between the three study groups showed no significant difference with respect to Gingival Index (GI), Simplified-Oral hygiene Index (OHI-S), bleeding index (BI), and Plaque Index (PI) at baseline. At 7 days post-intervention, Group C had the highest mean values of the all the four utilized parameters, that is, GI, OHI-S, BI, and PI scores when compared to both Group A (P = 0.001) and Group B (P < 0.001), while Group A and Group B did not differ significantly from each other in their improvement of clinical parameters at the 7th day of mouthwash usage (P = 0.52) [Graph 1].

- Post-intervention intergroup clinical analysis.
The intragroup comparison revealed that for both the test groups, namely, Group A and Group B the reduction in mean GI, OHI-S, PI and BI scores were significant at 7 days post-intervention when compared to baseline period at P < 0.001. On contrary, Group C did not show any significant change after the mouthwash use [Table 1].
| Parameters | Time | n | Mean | SD | Mean diff | P-value |
|---|---|---|---|---|---|---|
| GI | Baseline | 13 | 1.34 | 0.19 | 0.35 | <0.001* |
| 13 | 0.99 | 0.13 | ||||
| OHI-S | Baseline | 13 | 2.22 | 0.56 | 0.59 | <0.001* |
| 13 | 1.62 | 0.43 | ||||
| BI | Baseline | 13 | 1.86 | 0.55 | 0.49 | 0.001* |
| 13 | 1.38 | 0.42 | ||||
| PI | Baseline | 13 | 1.39 | 0.30 | 0.38 | <0.001* |
| 13 | 1.02 | 0.25 |
PI: Plaque index, GI: Gingival index, OHI-S: Simplified oral hygiene index, BI: Bleeding index, SD: Standard deviation
Microbial evaluation
Intergroup comparison demonstrated no significant difference observed concerning the mean Colony Forming Units (CFUs) for isolated microorganisms, S. mitis, S. salivarius, and A. actinomycetemcomitans between the three study groups at the baseline period. At 7 days post-intervention, the highest mean CFUs of S. mitis, S. salivarius, and A. actinomycetemcomitans was seen in Group C. However, between Groups A and B, the difference seen was not significant (P = 0.46) [Graph 2].

- Post-intervention microbiological analysis.
The intragroup comparison revealed that the mean CFUs of selected microorganisms in both Group A and Group B were significantly reduced at 7 days post-intervention, P < 0.001. Group C did not show any significant reduction after 7 days post-intervention [Table 2].
| Group | Time | n | Mean | SD | Mean diff | P-value | |
|---|---|---|---|---|---|---|---|
| Aerobic | Baseline | 13 | 161.38 | 53.99 | 60.38 | 0.001* | |
| 7 Days | 13 | 101.00 | 46.45 | ||||
| Anerobic | Baseline | 13 | 151.54 | 47.93 | 73.39 | 0.001* | |
| 7 Days | 13 | 78.15 | 40.34 | ||||
| Streptococcus mitis | Baseline | 13 | 8.38 | 7.72 | 6.00 | 0.003* | |
| 7 Days | 13 | 2.38 | 3.18 | ||||
| Streptococcus salivarius | Baseline | 13 | 11.38 | 7.85 | 8.00 | 0.002* | |
| 7 Days | 13 | 3.38 | 4.27 | ||||
| Aggregatibacter actinomycetemcomitans | Baseline | 13 | 33.08 | 17.97 | 18.54 | 0.001* | |
| 7 Days | 13 | 14.54 | 9.76 |
CFU: Colony-forming units, SD: Standard deviation
DISCUSSION
The persistent release of by-products by the aggregated bacteria in the biofilm produces the destruction of gingivae and periodontium. The mechanism of this destruction follows two different pathways, one is through oxidative damage, while another is through inflammatory mediators.[13,14] Condensed tannins, popularly known as proanthocyanidins, are present in GSE in eminent amounts, making them a rich source of phenolic compounds. They are the dimers or trimers of catechins and epicatechins. Gallic acid, gallocatechin, and epigallocatechin are other phenolic compounds that can be found in GSE.[15] One of the principal interactions of PAs is with proteins. The polyphenol-protein complexation is a “hand in glove” interaction and ascribes to its anti-inflammatory, antiadhesive, and cytoprotective effects.[16] Another key characteristic of PAs is their ability to scavenge oxygen and nitrogen radicals. V. vinifera has been proven to have greater antioxidant properties than vitamins C and E.[17] The results of the present study revealed that participants had no side effects of GSE mouthwash. They reported a puckering sensation in the mouth after using the mouthwash. Bennick 2002 states that this particular sensation can be attributed to the astringency produced due to the precipitation of salivary glycoproteins by condensed tannins and can be noted in other grape derived products such as grape juice and wine as well.[18]
The present study showed significant improvement while considering the four utilized clinical parameters, in both the test groups when compared to the control group. Although, insignificant chlorhexidine showed higher efficacy than the GSE mouthwash. This is attributed to the inhibition of extracellular and interstitial collagenase[7] strengthening of collagen by cross-linking,[2], and reduction of oxidative stress by the PAs present in GSE. Reduction in PI is due to the inhibition of enzyme GTF produced by initial colonizers of plaque. This enzyme helps in the production of insoluble glucans required for the cohesion of bacteria. GSE can inhibit the GTF by about 43.9%.[12] Rayyan et al., in 2006, reports similar findings, where GSE showed improvement in GI and PI.[7]
In this study, the placebo also reduced plaque, gingiva, and oral hygiene index to some extent, while no effect was seen for BI. This positive effect of placebo partly can be related to the Hawthorne effect.[19] Furthermore, the flushing effect due to rinsing with a placebo helps in the removal of food debris and material alba, interfering with the organization of the dental plaque.
Various in vitro studies with the hypothesis of GSE as an antimicrobial agent have been carried out, where GSE has shown an anti-bacterial effect against several periodontal pathogens. Mirkarimi et al., in 2013, report the Minimum Inhibitory Concentration (MIC) value of GSE against F. nucleatum and A. viscosus to be 2 mg/mL, and the Minimum bactericidal Concentration (MBC) values were double of that.[20]
Although required in a comparatively high dosage, GSEs are well tolerated by the human body without any negative ramifications, giving them an edge for clinical applications. Rayyan et al., in 2018, did the first clinical trial using 2% mucoadhesive GSE gel in the periodontal pockets by taking only clinical parameters for assessment, while the efficacy of GSE against periodontal pathogens was not analyzed.[7] Hence, in the present study, antimicrobial effect of GSE on supragingival pathogens was analyzed to assess its efficacy as an anti-plaque agent in comparison to chlorhexidine, the gold standard anti-plaque agent.
Both GSE and chlorhexidine showed a significant reduction in the selected periodontopathogens (P < 0.001). Between GSE mouthwash and chlorhexidine mouthwash, the difference was found to be insignificant (P = 0.46). Vanessa et al., in 2006, report similar results, where GSE showed significant antiplaque activity and inhibition of periodontal pathogens.[8] Haffajee et al., in 2008, also agree with the results of the present study as in their in vitro study similar results, in which GSE had less but comparable antimicrobial efficacy to chlorhexidine was seen.[21]
To the best of our knowledge, this is one the few clinical studies done to compare anti-inflammatory, antiplaque, and antimicrobial efficacy of GSE as compared to chlorhexidine in human participants. The limitation of the present study is that it is executed on a small pool of partakers and for a shorter intervention period, for it to give an absolute conclusive result.
CONCLUSION
GSE mouthwash showed similar efficacy as chlorhexidine in impeding the selected periodontal pathogens. The results of the present study indicate that the GSE mouthwash can be used as an efficacious supplement to oral mechanical cleaning. However, the scarcity of clinical trials depicts the lack of evidence-based effectiveness of this potent agent. This conveys the need for multicenter research with a large sample size which can lead to the subsequent development of GSE as a drug in periodontal therapy.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Financial support and sponsorship
Nil.
References
- Oral Health. 2012. Fact Sheet n 318. Available from: https://www.who.int/news-room/fact-sheets/detail/oral-health#:~:text=Periodontal%20(gum)%20disease&text=Severe%20periodontal%20diseases%20are%20estimated,oral%20hygiene%20and%20tobacco%20use [Last accessed on 2022 Mar 17]
- [Google Scholar]
- Grape seed extracts in dental therapy: Natural Oral Care in Dental Therapy United States: Wiley; 2020. p. :229-58.
- [CrossRef] [Google Scholar]
- Evidence-based update on diagnosis and management of gingivitis and periodontitis. Dent Clin N Am. 2019;63:69-81.
- [CrossRef] [PubMed] [Google Scholar]
- Evidence-based strategy for dental biofilms: Current evidence of mouthwashes on dental biofilm and gingivitis. Jpn Dent Sci Rev. 2019;55:33-40.
- [CrossRef] [PubMed] [Google Scholar]
- Short-term side effects of 0.2% alcohol-free chlorhexidine mouthrinse used as an adjunct to non-surgical periodontal treatment: A double-blind clinical study. J Periodontol. 2006;77:370-84.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy of grape seed extract gel in the treatment of chronic periodontitis: A randomized clinical study. J Invest Clin Dent. 2018;9:e12318.
- [CrossRef] [PubMed] [Google Scholar]
- Protective effects of grape seed proanthocyanidins against oxidative stress induced by lipopolysaccharides of periodontopathogens. J Periodontol. 2006;77:1371-9.
- [CrossRef] [PubMed] [Google Scholar]
- Sample size calculation for two independent groups: A useful rule of thumb. Proceed Singapore Healthc. 2011;20:138-40.
- [CrossRef] [Google Scholar]
- Comparative evaluation of chitosan chlorhexidine mouthwash in plaque control: A preliminary randomized controlled clinical trial. J Int Acad Periodontol. 2020;22:166-73.
- [Google Scholar]
- Comparative evaluation of clinical changes and microbial flora associated with usage of mouth washes containing green tea, chlorhexidine (0.2%) and essential oils in patients undergoing orthodontic therapy. Int J Drug Res Dent Sci. 2021;3:1-34.
- [Google Scholar]
- Preventive effects of an original combination of grape seed polyphenols with amine fluoride on dental biofilm formation and oxidative damage by oral bacteria. J Appl Microbiol. 2014;116:761.
- [CrossRef] [PubMed] [Google Scholar]
- Assessing the effect of pomegranate fruit seed extract mouthwash on dental plaque and gingival inflammation. J Dent Res Rev. 2016;3:117-23.
- [CrossRef] [Google Scholar]
- Modulation of the innate immune response within the periodontium. Periodontol 2000. 2004;35:53-74.
- [CrossRef] [PubMed] [Google Scholar]
- Procyanidin dimers and trimers from grape seeds. Phytochemistry. 1991;30:1259.
- [CrossRef] [Google Scholar]
- Characterization of protein-polyphenol interactions. Trends Food Sci Tech. 2004;15:186.
- [CrossRef] [Google Scholar]
- Oxygen free radical scavenging abilities of Vitamins C and E, and a grape seed proanthocyanidin extract in vitro. Res Commun Mol Pathol Pharmacol. 1997;95:179-89.
- [Google Scholar]
- Interaction of plant polyphenols with salivary proteins. Crit Rev Oral Biol Med. 2002;13:184.
- [CrossRef] [PubMed] [Google Scholar]
- The Hawthorne effect: A randomised, controlled trial. BMC Med Res Methodol. 2007;7:30.
- [CrossRef] [PubMed] [Google Scholar]
- The antimicrobial activity of grape seed extract against two important oral pathogens. Zahedan J Res Med Sci. 2013;15:43.
- [Google Scholar]
- Antimicrobial effectiveness of an herbal mouthrinse compared with an essential oil and a chlorhexidine mouthrinse. J Am Dent Assoc. 2008;139:606-61.
- [CrossRef] [PubMed] [Google Scholar]







