INTRODUCTION
Circulation crisis is a major feature of sepsis/septic shock and plays a pivotal role in this pathological process. This crisis results in a series of possible negative consequences, such as consecutive hypotension, multiple organ dysfunction syndrome and death. For the milestone showed in the end of 1870s by Louis Pasteur, patients present with puerperal septicemia after the evoking by microbes in blood. Together with all-relevant respects studied, increasing evidence has emerged that various pro-inflammatory mediators contribute to expand the pathological status caused by invading microorganisms. Therefore, cells sense external cues, i.e. a bewildering amount of pathogens, through receptors on their membrane surface, and then trigger intra-cellular signaling cascades transducing down and directing the cells to Hades-necrosis or/and apoptosis (programmed cell death).
A variety of molecules are involved in the downstream labyrinthian signaling pathways. Macrophage migration inhibitory factor (MIF), one versatile protein, influences several, even almost all of other transducing signals here or there, which consequently overbalances the systemic circulation and hemodynamic homeostasis. Here we summarize that how MIF plays a role as a molecular switch in sepsis/septic shock-related circulation collapse.
INTERACTION AMONG MIF AND OTHER MEDIATORS
Multifunctional cytokine MIF, as it has come to be known, exerts its original role in the immune system of organisms. Ubiquitously, MIF is expressed constitutively and secreted in a circadian manner in bodies. The effect of cytoplasmic and peripheral circulatory MIF on cardiovascular sensitivity to external environmental administration of vasoconstrictors or vasodilators is closely associated with a great number of respects. Wang described hypothetically that MIF exerts an unexpected role in the process of vascular hypo-reactivity during septic shock, and emerges as a “control point” molecule over this pathophysiological course [1]. In consistent with this, Larson described that MIF controls the neurohormonal response and the adaptive immune system, which results in the release of pro- and anti-inflammatory cytokines by controlling the balance of T-helper Th1 and Th2 lymphocytes function [2].
MIF, as an essential player in the onset-regulation of systemic vasoplegia, plays roles in changing vessel tone with or without the involvement of its tautomerase action in septic patients or models.
Nitric oxide (NO) is a highly reactive gas that can easily pass the lipid bilayer through a transmembrane manner, by which it is implicated in the vasodilation effect and in contribution to the host immunity [3]. The production of NO and its inducible nitric oxide synthase (iNOS) are consequently increased after inhibiting or blocking MIF during infection [4]. It is well accepted that apoptosis is involved in myocardial dysfunction and vascular paralysis in patients or rodents with sepsis [5-7]. p53, a fundamental tumor suppressor and apoptosis regulator, functions in inflammation by correlating with MIF, and thus results in pathological sequelae that contribute substantially to septic shock [8]. The complement system takes a crucial part in endotoxin-induced vasodilation, increased vascular permeability, and induction of cardiomyocyte apoptosis in sepsis. In addition, the process of MIF release strongly evoked by exposing to C5a via AKT phosphorylation by PI3K [9, 10].
The underlying basis of cellular reactivity in internal environment and microenvironment relies on a variety of positive ionic elements, known as cations. As Wang et al. showed that these major ions seem to play role as critical regulators through a complex network twisted with MIF, finally resulting in decrease in responsiveness to circulation active agents [1]. As it was known that insulin, an endocrine molecule, plays an important role in rhythmic vascular motion [11], and increasing data shows that insulin-induced change in vascular tone correlates with protein MIF [12], in addition to this, MIF can promote excessive release of insulin [13]. So the link of “MIF-insulin-vasodilation” formed in septic context and this finally results in consistent circulation dysfunction.
Initially, all types of vascular responsive aspects described above are up- or down-regulated by different intracellular signaling cascades evoked by the presence of enough inflammation-triggering molecules, such as lipopolysaccharide (LPS), in circulation. The complex intracellular signaling cross-talking network is, as Annane et al. illustrated, connected with various nuclear factors through several transducing steps and then linked finally with respective DNA domains after LPS’s binding to Toll-like receptor 4(TLR4) [14]. TLR4 unravelled early events in LPS induced signaling [15], and such transduction correlated with different pro-inflammatory cytokines [16].
From the beginning of the mesh between ligands and receptors to the production of different responsive molecular proteins into cytoplasm and blood, MIF always emerges as a regulator via interacting with most of them, such as TLR4, cytoplasmic phospholipase A2 (cPLA2), extracellular signal-regulated protein kinase (Erk), mitogen activated protein kinase (MAPK), phosphatidylinositol 3-kinase (PI3K), transcription factor E2F, inhibitory factor kinase1/2/3 (IKK1/2/3), activator protein-1 (AP-1), transcription factor Sp1 and NF-κB, and the serine/threonine protein kinase (Akt). [1, 14, 17, 18]. These signals mediate the upstream and downstream vasotonic regulatory proteins, and interestingly, MIF stands over these signaling molecules and switches them on or off (Figure 1).
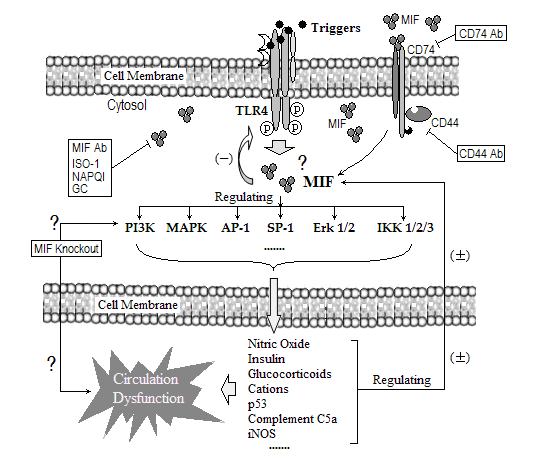 |
Further evidence has proved the switch role for MIF in the development of cardiovascular dysfunction in severe infectious conditions. We found that therapeutic MIF neutralization with MIF tautomerase inhibitor ISO-1 and MIF antibody reversed the cardiovascular dysfunction in cecal ligation and puncture (CLP)-induced sepsis, and correspondingly the survival rate got some improvement [19]. Chagnon et al [20] demonstrated that MIF neutralization reversed endotoxin-induced myocardial dysfunction in an experimental rodent model, during which MIF blockade with anti-MIF or isotypic-matched antibodies conferred the protection against sepsis by upregulating heart Bcl-2/Bax ratio that inhibits the release of cytochrome-c from mitochondria leading to less LPS-induced caspase-3 activity.
All these data are consistent with the results of Calandra et al that anti-MIF therapy protected the TNF-α-knockout mice from lethal peritonitis induced by both CLP and E coli, even when treatment was started up to 8 hours after CLP [21]. The increased resistance to the gram-negative bacterial product LPS, as well as the enhanced ability to clear P. aeruginosa infections in the lungs in MIF-knockout mice, indicate that neutralization or counteraction of MIF might be a key therapy for the treatment of circulation crisis in sepsis or septic shock [22]. Furthermore, MIF inhibition downregulates PI3K and its downstream target Akt, and positively mediates GATA-4 activity in cardiomyocytes, and then damages cardiac contractility in vivo. Meanwhile, MIF’s significant role in the late cardiac dysfunction related with burn injury was tested and found that it is often associated with microbes’ infection [23]. In addition, ISO-1, a tautomerase inhibitor of MIF, was used to reveal a similar effect as produced by using MIF antibody in cardiovascular crisis during the pathological state of sepsis/septic shock [19, 24].
Taken together, MIF appears like a primary pro-inflammatory protein staying at the switch position and determining the fate of cardiovascular cells by controlling series of signaling molecules that consequently causes systemic circulation paralysis in sepsis/septic shock.
TESTING OF MIF’S SWITCH ROLE IN SEPSIS-ASSOCIATED CIRCULATION CRISIS
Although MIF is considered as a switch center in circulation dysfunction in sepsis/septic shock, the testing ways to prove this thought is difficult. The interaction among various signaling factors is so complex that only limited steps and/or relative fewer items can be studied each time. While a deep exploration seems easy to be processed, the actual situation is always needed to be evaluated more extensively than the initial thought itself.
To prove the switch role of MIF in sepsis-related circulation crisis, the following steps may be useful. First, it is necessary to find certified evidence to prove the direct or indirect interrelationship between MIF and sepsis-related mortality or unacceptable outcomes. Moreover, an experimental environment or model as sepsis or septic shock should be produced in which only the intracellular signaling molecules are focused on, and this may be a clue for MIF’s influence on these signals in the process of sepsis-induced circulation collapse. Thirdly, studies can be designed to test MIF’s influence on circulation, which may be associated with serial cytoplasmic signals.
Because MIF exerts function through binding to its receptor molecule CD74 and then evokes the following intracellular signals, such as the rapid and transient activation of ERK pathway via CD74-dependent JAB1/CSN5 and Src kinase activity [18, 25-27], and the interaction between MIF and CD74 is accompanied with CD44 as a component of the MIF-CD74 receptor complex [28]. As thus, the effects of anti-CD74 and/or anti-CD44 on sepsis-associated circulation crisis and mortality should be evaluated, and it is worthwhile to explore the connection between CD74/CD44 and other previously mentioned signaling molecules. Finally, it should be understood clearly that it is difficult to say one molecule functions as a control center in serial pathophysiological processes. Even if MIF were a switch in sepsis-induced cardiovascular crisis, it is almost certain that interventions focus merely on MIF and cannot produce all expected results in the whole septic process, so with respect to the therapy of sepsis must be processed comprehensively. Therefore, a research-series should be figured out to guarantee MIF’s switch role in sepsis-associated circulation crisis.
CONCLUSION
Although the results and implications for clinical practice of this hypothesis are intriguing, it is still necessary to stimulate new studies designed to tease out the critical role of MIF in sepsis-related circulation crisis. Many studies have implied the contribution of this pluripotent cytokine MIF to the control of the occurrence of circulation collapse during sepsis/septic shock, but much of it are yet to be guaranteed. A notable thing should be concerned that MIF plays a switch role in triggering cardiovascular dysfunction that provides a fresh insight into the mechanisms that sculpt the progress in the pathological process of sepsis. If we can test MIF’s control role in sepsis-associated circulation crisis that have predictive value for defining clinical protective protocols, these could potentially be used in clinical practice and become a new clinical strategy for treating the circulatory collapse of this pathological process. Additional consideration should be given to this medical puzzle and a new vista should be opened in the future.
ACKNOWLEDGEMENT
We are indebted to Prof. Jie Song and Mrs. Cana Lee F. Laeno for their critical reading of the manuscript. We also thank Mrs. YuFeng Wang for her help in searching literatures.
LIST OF ABBREVIATIONS
Activator protein-1 (AP-1), Cecal ligation and puncture (CLP), Cytoplasmic phospholipase A2 (cPLA2), Extracellular signal-regulated protein kinase (Erk), Inducible nitric oxide synthase (iNOS), Inhibitory factor kinase1/2/3 (IKK1/2/3), Lipopolysaccharide (LPS), Macrophage migration inhibitory factor (MIF), Mitogen activated protein kinase (MAPK), Nuclear factor-kappa B (NF-κB), Nitric oxide (NO), Phosphatidylinositol 3-kinase (PI3K), Protein kinase Akt (Akt), T-helper 1 lymphocytes (Th1), Toll-like receptor 4(TLR4), Transcription factor E2F (E2F), Transcription factor Sp1 (SP1)
REFERENCES
- Wang F, Gao F, Jing L. Is macrophage migration inhibitory factor (MIF) the"control point"of vascular hypo-responsiveness in septic shock? Med Hypotheses. 2005 Aug 24;65(6):1082-7.
- Larson DF, Horak K. Macrophage migration inhibitory factor: controller of systemic inflammation. Crit Care. 2006 Apr 6;10(2):138.
- Ciornei CD, Egesten A, Engström M, et al. Bactericidal/permeability-increasing protein inhibits endotoxin-induced vascular nitric oxide synthesis. Acta Anaesthesiol Scand. 2002 Oct;46(9):1111-8.
- Koebernick H, Grode L, David JR, et al. Macrophage migration inhibitory factor (MIF) plays a pivotal role in immunity against Salmonella typhimurium. Proc Natl Acad Sci U S A. 2002 Oct 15;99(21):13681-6.
- Chopra M, Sharma AC. Apoptotic cardiomyocyte hypertrophy during sepsis and septic shock results from prolonged exposure to endothelin precursor. Front Biosci. 2007 May 1;12:3052-60.
- Matsuda N, Hattori Y. Vascular biology in sepsis: pathophysiological and therapeutic significance of vascular dysfunction. J Smooth Muscle Res. 2007 Aug;43(4):117-37.
- Chopra M, Sharma AC. Distinct cardiodynamic and molecular characteristics during early and late stages of sepsis-induced myocardial dysfunction. Life Sci. 2007 Jul 4;81(4):306-16.
- Fingerle-Rowson G, Petrenko O, Metz CN, et al. The p53-dependent effects of macrophage migration inhibitory factor revealed by gene targeting. Proc Natl Acad Sci U S A. 2003 Aug 5;100(16):9354-9.
- Riedemann NC, Guo RF, Ward PA. Novel strategies for the treatment of sepsis. Nat Med. 2003 May;9(5):517-24.
- Riedemann NC, Guo RF, Gao H, et al. Regulatory role of C5a on macrophage migration inhibitory factor release from neutrophils. J Immunol. 2004 Jul 15;173(2):1355-9.
- Hamburg NM, McMackin CJ, Huang AL, et al. Physical inactivity rapidly induces insulin resistance and microvascular dysfunction in healthy volunteers. Arterioscler Thromb Vasc Biol. 2007 Dec;27(12):2650-6.
- Tripathy D, Mohanty P, Dhindsa S, et al. Elevation of free fatty acids induces inflammation and impairs vascular reactivity in healthy subjects. Diabetes. 2003 Dec;52(12):2882-7.
- Waeber G, Calandra T, Bonny C, et al. A role for the endocrine and pro-inflammatory mediator MIF in the control of insulin secretion during stress. Diabetes Metab Res Rev. 1999 Jan-Feb;15(1):47-54.
- Annane D, Bellissant E, Cavaillon JM. Septic shock. Lancet. 2005 Jan 1-7;365(9453):63-78.
- Poltorak A, Smirnova I, He X, et al. Genetic and physical mapping of the Lps locus: identification of the toll-4 receptor as a candidate gene in the critical region. Blood Cells Mol Dis. 1998 Sep;24(3):340-55.
- Baumgarten G, Knuefermann P, Wrigge H, et al. Role of Toll-like receptor 4 for the pathogenesis of acute lung injury in Gram-negative sepsis. Eur J Anaesthesiol. 2006 Dec;23(12):1041-8.
- Roger T, David J, Glauser MP, et al. MIF regulates innate immune responses through modulation of Toll-like receptor 4. Nature. 2001 Dec 20-27;414(6866):920-4.
- Lue H, Kapurniotu A, Fingerle-Rowson G, et al. Rapid and transient activation of the ERK MAPK signalling pathway by macrophage migration inhibitory factor (MIF) and dependence on JAB1/CSN5 and Src kinase activity. Cell Signal. 2006 May;18(5):688-703.
- Wang F, Jing L, Chen J,et al. Role of macrophage migration inhibitory factor in septic shock-induced cardiovascular dysfunction: experiment with rats. Zhonghua Yi Xue Za Zhi. 2007 Mar 20;87(11):768-73.
- Chagnon F, Metz CN, Bucala R, et al. Endotoxin-induced myocardial dysfunction: effects of macrophage migration inhibitory factor neutralization. Circ Res. 2005 May 27;96(10):1095-102.
- Calandra T, Echtenacher B, Roy DL, et al. Protection from septic shock by neutralization of macrophage migration inhibitory factor. Nat Med. 2000 Feb;6(2):164-70.
- Bozza M, Satoskar AR, Lin G, et al. Targeted disruption of migration inhibitory factor gene reveals its critical role in sepsis. J Exp Med. 1999 Jan 18;189(2):341-6.
- Willis MS, Carlson DL, DiMaio JM, et al. Macrophage migration inhibitory factor mediates late cardiac dysfunction after burn injury. Am J Physiol Heart Circ Physiol. 2005 Feb;288(2) H795-H804.
- Al-Abed Y, Dabideen D, Aljabari B, et al. ISO-1 binding to the tautomerase active site of MIF inhibits its pro-inflammatory activity and increases survival in severe sepsis. J Biol Chem. 2005 Nov 4;280(44):36541-4.
- Leng L, Bucala R. Insight into the biology of macrophage migration inhibitory factor (MIF) revealed by the cloning of its cell surface receptor. Cell Res. 2006 Feb;16(2):162-8.
- Leng L, Metz CN, Fang Y, et al. MIF signal transduction initiated by binding to CD74. J Exp Med. 2003 Jun 2;197(11):1467-76.
- Cho Y, Jones BF, Vermeire JJ, et al. Structural and functional characterization of a secreted hookworm macrophage migration inhibitory factor (MIF) that interacts with the human MIF receptor CD74. J Biol Chem. 2007 Aug 10;282(32):23447-56.
- Shi X, Leng L, Wang T, et al. CD44 is the signaling component of the macrophage migration inhibitory factor-CD74 receptor complex. Immunity. 2006 Oct;25(4):595-606.