INTRODUCTION
Chlamydia trachomatis (CT) is one of the most common curable sexually transmitted organisms in the UK [1], with sixty four thousand new cases documented in 2000 by the PHLS [2], with an increase of 18% from the previous year. In the US, CT infection is the commonest cause of bacterial sexually transmitted infections with an estimated 3 million new cases annually. [3]
Clinical manifestations in males include urethral discharge, dysuria or urethral pruritus. On top, in females’ acute urethral syndrome, urethritis, bartholinitis, cervicitis, endometritis, pelvic inflammatory disease (PID), perihepatitis (Fitz-Hugh-Curtis syndrome) and reactive arthritis [4] can occur. Complications in females resulting from PID can lead to infertility which is also common in man, ectopic pregnancy and even chronic pelvic pain. While infection during pregnancy may result into premature rupture of membranes, preterm labour, neonatal death, postpartum endometritis and low birth weight. Transmission to the neonate is possible during delivery. Complications in neonates can be conjunctivitis, nasopharyngeal infections which can lead to pneumonia. Complications in males can be acute epididymitis, acute proctitis, and chronic prostatitis. Asymptomatic carriage can vary between 50% [5] and can affect as much as 88% [6] of the patients.
Recent study by McKay and coworkers [7] have shown that the young females in the UK were more likely to be infected with Chlamydia trachomatis, than males and their findings have shown that the prevalence was much higher than those cited before. It is estimated that 1% of females in the age bracket of 16 to 19 years were diagnosed with genital chlamydial infection in a GUM clinic in the UK. It has been suggested that younger females are more prone to chlamydial infection due to their immunity and also due to the viscosity of the mucous lining in their cervix. Only recently Mcmillan and colleagues [8] concluded that the prevalence of chlamydial infection females is age related irrespective of clinical presentation and that sexually active females less than 30 years old should be targeted. An annual cost of $2 billion is required for the treatment of chlamydial infections and its complications.
CT are obligate intracellular, gram-negative bacteria with a dimorphic developmental cycle that takes place entirely within a membrane-bound vacuole termed an inclusion.The process used by chlamydiae to enter cells is not fully understood. However, a receptor-mediated process is thought to be responsible for this process. [9] The organism is internalized by clathrin coated pits. Work by Kaushic et al [10], showed that the antigenic presentation, IgA, transport and presence of immune cells in the uterus and vagina are under organ specific control. They were able to show in their rat model, that progesterone increases and oestradiol decreases the susceptibility to intrauterine chlamydial infection. They further hypothesised that, under the influence of progesterone, genital tract epithelial lining is thinned. In this process, it was postulated that there might be a differential expression of receptors in the genital lining allowing initiation of contact between chlamydia and the host epithelial cells.
It was originally thought that Chlamydia spp lack the ability to synthesize ATP to support their growth and reproduction and could hence reproduce only within compatible host cells. However, this view of Chlamydia spp as ‘enzyme-parasites’ was only supported by indirect evidence [11]. More recently, Iliffe-Lee and McClarty [12] identified and characterized chlamydial glucose-catabolising enzymes indicating that Chlamydia spp contain complete functional Embden-Meyerhof-Parnas and/or Entner-Doudoroff pathways and may contain a complete pentose phosphate pathway. Furthermore, the recent sequencing of C.trachomatis serovar D indicates that Chlamydia genome encodes for several energy-producing enzymes [13]. If Chlamydia spp do indeed scavenge ATP from the host cell this may be only necessary early in the infectious process before the Chlamydia spp have synthesized the full complement of enzymes necessary to generate ATP, endogenously through glycolysis and the pentose phosphate pathway [14]. CT an intracellular parasite is thought to produce several glycosidase enzymes. These enzymes have been shown to hydrolyse heparan sulphate, which consists of N-acetylated or N sulphated glucosamine or galactosamine linked to glucoronic or iduronic acid. Extensive work has been done by Connaris and Greenwell [15], who demonstrated that both Trichomonas vaginalis (TV) and Tritrichomonas foetus possess a wide range of intracellular glycosidases. On determining the activity of this group of enzyme, they found that Beta galactosidase, α N acetyl galactosaminidase and β –N acetyl glucosaminidase were the most active. These enzymes have the ability to metabolise mucin, constituting the protective mucus layer of the female genital tract. Mucin is a high molecular weight glycoprotein comprising of 80% carbohydrate has been shown to be metabolized by TV as either a means of obtaining energy or as part of a pathological process [15]. The enzyme β galactosidase has been successfully purified by Vella and Greenwell [16].
Glycosides are aldehyde derivative of a six membered ring structure formed by the reaction of alcoholic hydroxyl group at carbon atom 5 with the aldehydic carbon atom [1].
Forming a six membered ring sugar termed a pyranose; a derivative from a heterocyclic compound pyran. Pyranoses are formed by the; reaction between an alcohol and an aldehyde forming a hemiacetal. This hemiacetal contains an asymmetric carbon atom and therefore can exist in two stereo isomeric form. These are designed as α and β. These aldo pyranose can readily react with alcohols in the presence of a mineral acid to form anomeric α and β glycosides. These glycosides differ from each other in configuration about the carbonyl carbon atom. These sugar molecules are; hydrolysed by a group of enzymes; the glycosidases. These are enzymes that specifically cleave glycosidic bond linking the pyranose and the hydroxyl group furnished by an alcohol.
However on surveying the literature through Pubmed [17-19], no work has been reported associated with glycosidases activity in CT. These group of enzymes activity has therefore been studied in this research.
MATERIAL AND METHOD
In this study, CT SA2F a LGV2 strain [20] was used. This strain that was provided by the Chlamydia Department of University College London Hospital (UCLH), London. This serotype has been extensively used and passaged at UCLH.
Detection of Glycosidase activity
Glycosidases activity in trichomonads has been studied by Connaris and Greenwell [15] using synthetic substrates derivatives of p-nitrophenol (pNP). This test was described by Kilian and Bulow [21]. The different glycoside sugars tested are linked to a p-NP (para-nitrophenol) molecule. On enzyme hydrolysis the pNP molecule is released and forms a yellow product. The reaction is stopped to enhance the substrate colour and the end product can be measured by absorbance spectrophotometry at 405nm. This method was adopted to measure the activity of glycosidases in CT.
Preparation of McCoy cells for CT sub culturing
McCoy cells were grown in 15ml of Minimum Essential Medium (MEM) supplemented with 5% foetal calf serum (FCS), 2% essential amino acids, 1% vitamin, 10µg/ml gentamicin and 2.5 µg/ml amphotericin B (all products from Sigma Diagnostics) in 25 cm2 tissue culture flasks (Helena Biosciences) at a concentration of 2x105 cells per ml at 37ºC in 5% CO2 for 48h or until confluency. The growth media was then replaced with fresh growth media containing 2 µg/ml of cycloheximide and 0.5% glucose. The culture flasks were seeded with 1ml CT inlusion bodies. The flasks were spun at 2500 rpm in a microtitre plate centrifuge for 1.5 h at room temperature (RT) to increase the infection rate by the CT. The flasks were incubated at 37ºC in 5% CO2 for 48h. The infected McCoy cells were scraped from the culture flask using a plastic baton. The contents of the flask were homogenized in situ and the flask was transferred to an ice bucket. The inclusion bodies were released from the infected McCoy cells by a method described by Schachter and Wyrick [22] using a sonicator probe (MS 73, Phillip Harris Scientific). The contents of each flask were transferred into 50 ml centrifuge tubes which were spun at at 2500 rpm for 10 min. The supernatant was then transferred into a 50 ml Oak ridge PPCO centrifuge tube (Nalge, USA), leaving behind a cell debris pellet. The tubes were spun at 12000 rpm at 4ºC in a fixed rotor centrifuge for 1 h to allow deposition of inclusion bodies. After centrifugation, the supernatant was discarded; the inclusion body pellet was washed briefly in PBS, then reconstituted in 2.0 ml of PBS. An inclusion body count was then performed; 50 µl aliquot was removed and transferred onto a microscope slide containing a Teflon ring. The slide was allowed to dry, by heating on a slide dryer. The slide was then flooded with 5% Lugol's iodine for 10 min., blotted dry and an inclusion count was carried out. CT's inclusion bodies contain glycogen that takes up the iodine and forms a dark brown mass that is visible under light microscope. The slides were visualized under oil immersion and an inclusion bodies count was performed for future assays, which were concentration dependent. Citrate- phosphate buffers at; pH 5.0, 5.5, 6.0, 6.5, 7.0, 7.5 and pH 8.0 were prepared to determine the activities of the glycosidases on the above substrates.
To 25µl of inclusion body lysate, 20 µl of pNP sugar and 10 µl of 0.05 M buffer were added. The contents of the microtitre plate were mixed on a plate mixer, sealed with a plastic seal and incubated at 37ºC at three different time intervals, 16h, 8h and 4 h. After incubation, the reaction was stopped with 55µl 3M NaOH. The plates were then read at 405 nm in a microtitre plate reader. The assays that were performed in microtitre plates over a range of pHs from 5.0 to 8.0. 25µl of CT lysate (with or without prior treatment with triton) , 20µl of pNP sugars, 10µl of 0.05M citrate-phosphate buffer were added to each well and the microtitre plates were incubated at 4h, 8h and 15h intervals at 37ºC . The reactions were then stopped with the addition of 55µl of 3M NaOH (120g/L) and the plates were read at 405 nm in a Dynatech MRX.
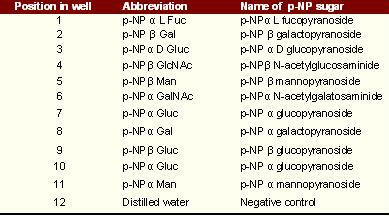 |
Content of microtitre plate layout
Microtitre plates were used and the rows were labelled corresponding to a different pH, while the column was for each pNP sugar. The last column was used for negative control.
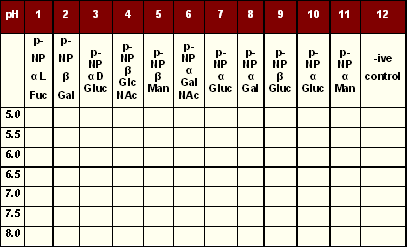 |
Layout of p-NP sugars and buffers in microtitre plate |
To 25μl of inclusion body lysate, 20μl of pNP sugar and 10μl of 0.05 M buffer were added. The contents of the microtitre plate were mixed on a plate mixer, sealed with a plastic seal and incubated at 37°C at three different time intervals, 16h, 8h and 4 h. After incubation, the reaction was stopped with 55μl 3M NaOH. The plates were then read at 405 nm in a microtitre plate reader.
The experiment was performed on 4 different occasions and in all instances were performed in duplicate. Similar result patterns were obtained. These experiments were also conducted at 4h, 8h and12 h. The enzymatic hydrolysis of different pNP substrates was assessed. The different enzymatic activity at each incubation time is represented by the absorbance value.
RESULTS
The absorbance read at 405 nm was expressed as activity of the different enzymes in pNP ((M) produced. This method was used according to Calibration curve by Vella [23]. However, the graph had to be extended to cover the range of absorbance in this experiment. Tables and show amount of pNP M produced on degradation of pNP sugars per 25µl enzyme with and without triton lysis. The amount of pNP produced is equivalent to the amount of sugar degraded.
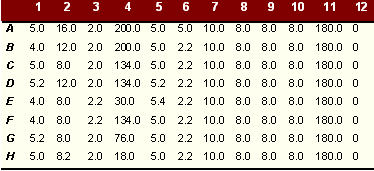 |
Activity of different glycosidases extracted from CT after triton digest over a range of pHs after overnight incubation |
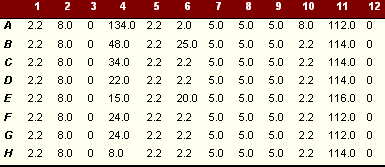 |
Activity of different glycosidases extracted from CT without triton digest over a range of pH after overnight incubation |
These tables show amount of pNP (mm) produced on degradation of pNP sugars per 25µl enzyme with and without triton lysis. The amount of pNP produced is equivalent to the amount of sugar degraded. At pH of 5.0 the enzymatic activity in the inclusion bodies was highest. The pattern of activity was similar at different pHs. As shown, there was a considerable difference in enzyme activity between triton extracted and untreated reticulate bodies. The results imply that some of the enzyme is either soluble or present in an active state on the bacterial surface (about 75% of the activity demonstrated) and some (about 25%) is membrane bound and freed by triton extraction. It should be noted that the triton extracted material is a mixture of both soluble and solubilised material.
Similar enzymatic activities were detected using p-NP a mannopyranoside as substrate. Although in this case most of the mannosidase recovered (88%) was readily soluble with little extra enzyme activity (12%) released on triton treatment. This finding was reflected in readings at other pHs at which the enzyme was tested.
After baseline blanking, activity against pNP ß-N acetylglucosamine and pNP a mannopyranoside were the highest although the activity against a glucopyranoside and ß galactopyranoside were also high. Enzymatic activity using other p-NP sugars was negligible over the 12 h testing period.
DISCUSSION
Little is known about the mechanism by which CT digest the protective mucous found in the cervix and infect the epithelial cells. One possible way could be through glycosidase activity. Enzymatic activity expressed against different p-NP sugars suggests that CT could compromise the mucous barrier of the cervix that prevent bacterial colonization of the cervical cells by removal of the carbohydrate molecules which give mucous its gel-like qualities.
N- acetylglucosamine and D-glucuronic acid make up hyaluronic acid, a simple acid mucopolysaccharide which is a major component of the intracellular filler between cells. [24] While mannose in the form of GDP-mannose reacts with phosphoryldolichol to give mannosyl phosphodolichol. Dolichols are found either free or phosphorylated (phosphodolichol) in the endoplasmic reticulum and golgi apparatus, but not in the plasma or mitochondrial membranes. [24] CT may act on the hyaluronic acid found between cells, damaging the plasma membrane and allowing bacterial invasion of host cells.
Another interesting finding is the enzymatic activity at a pH between 5.0 and 5.5 is significantly high. The pH of the vagina is between a pH range of 5.0 to 5.5, suggesting that the activity of these enzyme would be optimal in such an environment.
Activity against β-N acetylglucosamide and α mannopyranoside , using para nitrophenol (pNP) conjugates showed that chlamydia expressed high levels of β-N acetylglucosaminidase and α mannosidase. These glycosides are components of hyaluronic acid found as extracellular matrix and mannosyl phosphodolichol, found in endoplasmic reticulum respectively. Interestingly, the cysteine rich major outer membrane protein (MOMP), having a molecular mass of approximately 40 KDa is glycosylated containing N-linked carbohydrate residues, which can bind to concanavalin A, wheat germ agglutinin and Dolichos biflorus. These lectins are known to recognize α-D mannose, β-N acetylglucosamine, sialic acid and β-N acetylgalactosamine.
Swanson and Kuo [25], were able to competitively prevent binding of the organisms to HeLa cells by introducing these sugars, with a maximum inhibition of 73% with mannose, 85% with galactose and 79% with N-acetylglucosamine. With the presence of a glycosylated MOMP, the CT is likely to avoid detection by the PMNL when they are present outside cells as they will be recognized as part of the hyaluronic acid in the intracellular filler found between cells. Spread of chlamydia can then be mediated canicularly along adjacent epithelia through the movement of elementary bodies via the apical surface of mucosal epithelia and also in joints. Olmez et al [26], were able to demonstrate the presence of CT DNA in the synovial fluid of patients with osteoarthritis. The presence of CT can be used as a surrogate marker in cervical cancer for HPV. Allderling, Jordan and Boardman [27] were able to detect a higher rate of progression to CIN 3 in abnormal cervical cytology changes suggestive of CT. If CT does not directly influence the direct progression of a cervical neoplasia, it may interfere with the balance of the local immunity, allowing other agents like HPV to infect epithelial cells and cause mutations.
Although high levels of b-N acetylglucosaminidase and a-mannosidase were detected, a bioinformatics search of the whole genome published for chlamydia, failed to detect b-N-acetylglucosaminidase or a- mannosidase. So far only 70% of chlamydial genes have been characterized and allocated function. The only related carbohydrate metabolising enzymes identified were amylase and amylomaltase, b- N-acetyl glucosaminyl transferase and a mannosyl transferase. This search was performed by downloading sequence data on known bacterial glycosidases and performing a manual search for conserved areas using the ClustalW algorithm for sequence alignment and BlastP for protein homology searches and then the chlamydia genome was interogated for signature sequences in order to identify the enzymes. Interestingly, McCoy et al [6], undertook a genetics based approach, characrerised a gene similar that was MurA, a UDP-N-acetylglucosamine enolpyruvyl transferase that catalyzes the first committed step of peptidoglycan synthesis that is associated with chlamydial anomaly. DNA sequence analysis of murA from C. trachomatis predicted a cysteine-to-aspartate change in a key residue within the active site of MurA. This sort of amino acid change in CT could account for the minimal homology was seen in the genome for either enzyme.
CONCLUSION
Homology between α mannosidases and in β-N acetylglucosaminidases between species is low. Although the genome of C trachomatis has been sequenced, only 70% of the genome has been assigned any function. It was very difficult to identify any genes that coded for these enzymes. There may also be some divergence in the different chlamydial serotypes; hence the gene for these enzymes can vary between serogroups. Clearly to derive full sequence information, the enzymes must be purified and information derived from limited N-terminal sequencing used to interrogate the genome again. Knowledge of the sequence could allow the production of antisense oligonucleotides for use therapeutically.
REFERENCES
-
Perkins E, Carlisle C, Jackson N. Opportunistic screening for Chlamydia in general practice: the experience of health professionals. Health Soc Care Community 2003;11(4):314-20.
-
Communicable Disease Report. CDR Weekly 31 March 2000.
-
Sexually Transmitted disease surveillance 2001. CDC 2002. Atlanta. Centers for Disease Control and Prevention Sep 2002.
-
Stamm WE. Chlamydia trachomatis infections in the adult. Holmes KK, Mardh PA, Sparling PF, et al Eds. Sexually transmitted disease 3rd ed. 1999. New York Mc Graw-Hill.
-
Dixon L, Pearson S, Clutterbuck DJ. Chlamydia trachomatis infection and non-gonococcal urethritis in homosexual and heterosexual men in Edinburgh. Int J STD AIDS 2002;13(6):425-6.
-
McCoy AJ, Sandlin RC, Maurelli AT. In vitro and in vivo functional activity of Chlamydia MurA, a UDP-N-acetylglucosamine enolpyruvyl transferase involved in peptidoglycan synthesis and fosfomycin resistance. J Bacteriol 2003;185(4):1218-28.
-
McKay L, Clery H, Carrick-Anderson K, Hollis S, Scott G. Genital Chlamydia trachomatis infection in a subgroup of young men in the UK. Lancet 2003;361(9371):1792.
-
Macmillan S, McKenzie H, Flett G, Templeton A. Which women should be tested for Chlamydia trachomatis? BJOG 2000;107(9):1088-93.
-
Jutras I, Abrami L, Dautry-Varsat A. Entry of the lymphogranuloma venereum strain of Chlamydia trachomatis into host cells involves cholesterol-rich membrane domains. Infect Immun 2003;71(1):260-6.
-
Kaushic C, Zhou F, Murdin AD, Wira CR. Effects of Estradiol and progesterone on susceptibility and early immune responses to Chlamydia trachomatis infection in the female reproductive tract. Infect. Immun 2000;68(7):4207-16.
-
Atlas RM. Principles of Microbiology. 2nd edition (international) 1997. WCB McGraw-Hill, Boston. 994-7.
-
Lliffe-Lee ER, McClarty G. Glucose metabolism in Chlamydia trachomatis: the “energy parasite” hypothesis revisited. Mol Microbiol 1999;33(1):177-87.
-
Stephens RS, Kalman S, Lammel C, Fan J, Marathe R, Aravind L, Mitchell W, Olinger L, Tahusor RL, Zhao Q, Koonin EV, Davis RW. Genome sequence of an obligate intracellular pathogen of humans: Chlamydia trachomatis. Science 1998;282(5389):754-9.
-
Shaw EI, Dooley CA, Fischer ER, Scidmore MA, Fields KA, Hackstadt. Three temporal classes of gene expression during the Chlamydia trachomatis developmental cycle. Mol Microb 2000;37(4):913-925.
-
Connaris S, Greenwell P. Glycosidases in mucin-dwelling protozoans. Glycoconj J 1997;14(7):879-82.
-
Vella M, Greenwell P. Purification and partial characterization of beta-galactosidase from Tritrichomonas foetus. Glycoconj J 1997;14(7):883-7.
-
http://www.ncbi.nlm.nih.gov/BLAST
-
http://www.tigr.org
-
http://clustalw.genome.ad.jp
-
Morrissey I, Salman H, Bakker S, Farrell D, Bebear CM, Ridgway G. Serial passage of Chlamydia spp. in sub-inhibitory fluoroquinolone concentrations. J. Antimicrob. Chemother 2002;49(5):757-61.
-
Kilian M, Bulow P. Rapid diagnosis of enterobacteriaceae. Detection of bacterial glycosidases. Acta. Pathol. Microbiol. Scand. [B] 1976;84B(5):245-51.
-
Schachter J, Wyrick PB. Cultivation and Isolation of Chlamydia trachomatis. Methods Enzymol 1994;236:377-90.
-
Vella M. Biochemical and Molecular Characterisation of an Enzyme β- galactosidase from the protozoan Tritrichomonas foetus. PhD Thesis. 1998. University of Westminster. UK.
-
Lehninger AL. Biochemistry. 2nd Ed. 1981. Worth. New York USA. 256-76.
-
Swanson AF, Kuo C. Binding of the glycan of the Major Outer Membrane Protein of Chlamydia trachomatis to HeLa cells. Infect. Immun 1994;62(1):24-8.
-
Olmez N, Wang GF, Li Y, Zhang H, Schumacher HR. Chlamydial nucleic acids in synovium in osteoarthritis: what are the implications. J Rheumatol 2001;28(8):1874-80.
-
Allderling T, Jordan S, Boardman R. Association of human papillomavirus and chlamydia infections with incidence of cervical neoplasia. Acta Cytol 1985;29:653-60.