INTRODUCTION
Stem cells are primal cells common to all multicellular organisms that retain the ability to renew themselves through cell division and can be differentiated into a wide range of specialized cell types. Modern therapeutics is having a lot of hope from stem cell research in the field of organ transplantation and replacement of lost tissue. By virtue of self renewal and potency, stem cells can form various types of tissue cells. The regulators of stem cell growth at genomic and proteomic level are identified and we might be able to control stem cell in vitro. In developed countries, stem cell transplant has become a therapeutic option but in developing countries, it is still under trial phase. There can be two sources of stem cells – Autologous and Allogenic. Autologous embryonic stem cells generated through therapeutic cloning and highly plastic adult stem cells from the umbilical cord blood or bone marrow are promising candidates. Allogenic stem cells can be derived from marrow, peripheral blood, cord blood, family donors or HLA typed or untyped unrelated donors. This article focuses on types of stem cells and stem cell regulation with enlightening comments on clinical application and future aspects.
HISTORICAL BACKGROUND
Although the first attempts were made to fertilize mammalian eggs outside the body in 1878, research in human stem cell field grew out of findings by Canadian scientists Ernest A. McCulloch and James E. Till in the 1960s [1, 2]. The first use of bone marrow transplant in the present context to stem cell transplant (SCT) was done by Schretzenmyr in 1937 [3] as these stem cells are known to be present in the bone marrow of adults. [4] First animal made by in-vitro fertilization (IVF) in 1959 was also a step towards SCT. In late 1960s, teratocarcinomas were determined to originate from embryonic germ cells in mice and Embryonal Carcinoma (EC) cells were identified as a kind of stem cell. The first human egg was fertilized in vitro in 1968 and raised the possibility of exploitation of totipotency of stem cells. Cultured EC cells were explored as models of embryonic development in mice in 1970s. In 1981, it was proved that mouse Embryonic Stem (ES) cells are derived from the inner cell mass of blastocysts. Mouse ES cells were grown in vitro and ES cells injected into mice which formed teratomas. Between 1984-1988 pluripotent clonal cells called Embryonal Carcinoma (EC) cells were developed. When exposed to retinoic acid these cells differentiated into neuron-like cells and other cell types. A clonal line of human embryonal carcinoma cells was derived that yields tissues from all three primary germ layers in 1989. They had limited replicative and differentiative capacity. In 1994, human blastocysts were generated and the inner cell mass was maintained in culture. Cells like ES cells formed in the center and retained stem cell like morphology. In 1995-96, non-human primate ES cells were maintained in vitro from the inner cell mass of monkeys. These cells were pluripotent and differentiated normally into all three primary germ layers [3].
Embryonic Stem cells (ES) cells from the inner cell mass of normal human blastocysts were cultured and maintained normally for many passages in 1998. In 2000, scientists derived human ES cells from the inner cell mass of blastocysts. They proliferated in vitro for a long time and form all three germ layers and teratomas when injected into immune deficient mice. The onset of 21st century hampered the stem cell research due to changed US funding rules; however the funding from The California Institute for Regenerative Medicine supported the research. Stem cell research became more promising as human ES cell lines were shared and new lines were derived, more research groups were focusing attention on the differentiation of cells in vitro.
WHAT IS STEM CELL?
Stem cells are primal cells which are considered to be progenitor of more than 200 cell types present in adult body. All stem cells are unspecialized (undifferentiated) cells that are characteristically of the same family type (lineage). They retain the ability to divide throughout life and give rise to cells that can become highly specialized and take the place of cells that die or are lost.
The rigorous definition of a stem cell requires that it possesses two properties: Self renewal and Unlimited potency. Self renewal means the ability to go through numerous cycles of cell division while maintaining the undifferentiated state. Unlimited potency means the capacity to differentiate into any mature cell type. In a strict sense, this makes stem cells either totipotent or pleuripotent. Multipotent and unipotent are also described to define stem cell potency. These properties can be illustrated in vitro using methods such as clonogenic arrays where the progeny of cells is characterized [5].
Two broad categories of stem cells exist: embryonic stem cells derived from blastocyst and adult stem cells which are found in adult tissue. In a developing embryo, stem cells are able to differentiate into all the specialized embryonic tissue. In adults, stem cells act as a repair system for the body replacing specialized damaged cells.
POTENCY DEFINITIONS
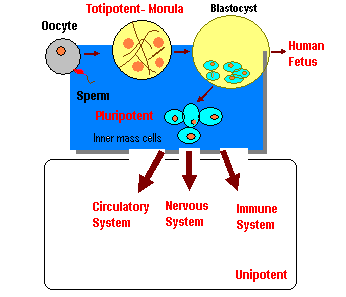
Potency specifies the differential potential of the stem cells. Totipotent stem cells are produced from the fusion of an egg and a sperm cell. Cells produced by the first few divisions of the fertilized egg are also totipotent. These cells can differentiate into embryonic and extraembryonic cell types. Only the morula cells are totipotent able to become all tissues including a placenta. Pleuripotent stem cells are the descendents of totipotent cells and can differentiate into cells derived from 3 germ layers. Pleuripotent stem cells originate as inner cell mass within a blastocyst (Blastula). Blastocyst is a thin walled hollow sphere made up of an outer layer of cells, a fluid filled cavity and an inner cell mass containing pleuripotent stem cells. The blastocyst develops after cleavage and prior to implantation, in approximately 5 days. These stem cells become any type of tissue in the body excluding a placenta. Multipotent stem cells can produce only cells of a closely related family of cells e.g. hematopoetic stem cells differentiate into red blood cells, white blood cells, platelets etc. Unipotent stem cells can produce only one cell type but have the property of self renewal which distinguishes them from nonstem cells.
 |
TYPES OF STEM CELLS
Stem cells are broadly classified into two categories: Embryonic stem cells (ESC) and Adult stem cells (ASC).
Embryonic Stem Cells
These cells are also known as early stem cells. Embryonic stem cells are derived from embryos at a developmental stage before the time of implantation would normally occur in the uterus. This developmental stage is the blastocyst stage – 32 cell stage, from which these pleuripotent cells can be isolated [6].
Pleuripotency of embryonic stem cells: Embryonic stem cells can give rise to cells from all three embryonic germ layers i.e. ectoderm, mesoderm and endoderm, even after being grown in culture for a long time. In other words they can develop into each of more than 220 cell types of the adult body when given the sufficient and necessary stimulation for a specific cell type. ES cells can be maintained in culture as undifferentiated cell lines or induced to differentiate into many different lineages [7]. Pleuripotency distinguishes ES cells from multipotent cells found in adults, which can only form a limited number of different cell types.
Regulation of pleuripotency of ES cells: Researches at Genomic institute, Singapore in collaboration with colleagues from US, have discovered a gene that plays a crucial role in human embryonic stem cells. Scientists studying on mice identified a gene that encodes a transcripion factor, Sall4, a protein that switches gene on and off. Such transcription factors are crucial for the identity of the cell. Transcription factors also regulate the development of cells from the primitive cell stage to functional cell making up the tissue and entire development from the fertilized egg to grown individuals. There are various proteins described which regulate the pleuripotency of ES cells. Some of these are:
-
Oct 4 protein: It has been used as a key marker for ES cells and for the pleuripotent cells of the intact embryo. Its expression must be maintained at a critical level for ES cells to remain undifferentiated.
-
Nanong protein: It is essential for maintenance of the undifferentiated state of the mouse cells. The expression of Nanong decreased rapidly as mouse ES cells differentiated and when its expression level was maintained by a constitutive promoter, mouse ES cells could remain undifferentiated and proliferate in the absence of either LIF or BMP in serum free medium. Nanong is also expressed in human ES cells, though at a much lower level compared to that of Oct4 and its function in human ES cells was yet to be examined. Recent studies also implicate the Wnt-b-catenin signaling in maintaining pleuripotency [8].
Adult Stem Cells
Adult stem cells are undifferentiated cells found through out the body that divide to replenish dying cells and regenerate damaged tissue. They are also known as somatic stem cells which can be found in children as well as adults.
Properties: The rigorous definition of stem cell require that it possesses two properties: Self renewal- the ability to go through numerous cycles of cell division while maintaining the undifferentiated state and Multipotency- the ability to generate progeny of several distinct cell type e.g. both glial cells and neurons, opposed to unipotency restriction to a single cell type. To ensure self renewal, stem cell undergoes two types of cell division: symmetric division give rise to two identical daughter cells both endured with stem cell properties and asymmetric division which produces only one stem cell and a progenitor cell roperties and asymmetric division which produces only one stem cell and a progenitor cell with limited self renewal potential. Progenitor can go through several round of cell division before terminally differentiating into a mature cell. It is believed that molecular distinction between symmetric and asymmetric division lies in differential segregation of cell membrane proteins (such as receptors) between the daughter cells.
Regulation of differentiations of Adult Stem Cells: Adult stem cell researches have been focused on uncovering the general molecule mechanism that control their self renewal and differentiation.
-
Bmi-1: The transcriptional repressor Bmi-1 is one of the polycamb-group proteins, which was discovered as a common oncogene activated in lymphoma [9] and later shown to specially regulate hemato-poietic stem cells [10]. The role of Bmi-1 has also been illustrated in neural stem cells [9].
-
Notch: The Notch pathway has been known to developmental biologists for decades. Its role in control of stem cell proliferation has now been demonstrated for several cell types including hematopoietic, neural and mammary stem cells [11].
-
Sonic hedgehog and Wnt: These developmental pathways are also strongly implicated as stem cell regulators [12].
-
Plasticity: A change in stem cell differentiation from one cell types to another is called trans differentiation, and the multiplicity of stem cell differentiation options is known as developmental plasticity [13, 14].
Type of Adult Stem Cells: Stem cells with broad differentiation potential appear to exist in adult bone marrow and, perhaps, in other tissues as well. Stem cells located outside of the bone marrow are generally referred to as tissue stem cells. Such stem cells are located in sites called niches [15] (niche- a specialized cellular environment that provides stem cells with the support needed for self-renewal. Straddling and Xie characterized the niche cells that govern the production of Drosophila embryonic germline stem cells- those cells in the ovary that are the earliest precursors to eggs. According to the scientists, their findings offer a potentially valuable model to explore how stem cells are regulated in vivo). For instance in the gastrointestinal tract they are located at isthmus of stomach glands and at the base of crypts of the colon. Niches have been identified in other tissues, such as the bulge area of hair follicles and the limbus of cornea [16-18].
-
Bone marrow stem cells: Bone marrow is the major source of adult stem cells. There are mainly two types of marrow stem cells: Bone marrow hematopoietic stem cells: Hematopoietic stem cells are stem cells and the early precursor cells which give rise to all the blood cell types that includes both the myeloid (monocytes and macrophages, neutrophils, basophils, eosinophils, erythrocytes, megakaryocytes/platelets and some dendritic cells) and lymphoid lineages (T-cells, B-cells, NK cells, some dendritic cells). Hematopoietic stem cells generate all the blood cells and can reconstitute the bone marrow after depletion caused by disease or irradiation [19, 20]. Bone marrow stromal stem cells: Mammary stem cells provide the source of cells for growth of mammary gland during puberty and gestation and play an important role in carcinogenesis of breast [21]. A single such cell can give rise to both luminal and myoepithelial cell types of the gland and has been shown to regenerate the entire organ in mouse [22]. Mesenchymal stem cells are multipotent stem cells that can differentiate into variety of cell types in vitro or vivo include osteoblasts, chandrocytes, myocytes, adipocytes, neuronal cells, and described lately, into beta pancreatic ielet cells. These cells have been classically obtained from the bone marrow, and mesenchymal stem cells can some time refer to marrow stromal cells. While the terms mesenchymal stem cell and stromal cells have been used interchangeably, they are increasingly recognized as separate entities as: Mesenchymal stem cells can encompass multipotent cells derived from other non-marrow tissues, such as adult muscle side population cells or the Wharton’s jelly present in the umbilical card; and Stromal cells on a highly heterogenous cells population consist of multiple cell types with different potential for proliferation and differentiations. In contrast, Mesenchymal stem cells represent a more homogenous subpopulation of mononuclear progenitor cells possessing stem cells features specific cell surface markers.
-
Neural stem cells: The existence of stem cells in the adult brain has been postulated following the discovery that the process of neurogenesis, birth of new neurons, continues into adulthood in rats. Normally adult neurogenesis is restricted to the subventriculare zone, which lines the lateral ventricles of the brain, and the dentate gyrus of the hippocampal formations. Although the generator of new neurons in the hippocampus is well established, the presence of true self renewing stem cells there has been debated [23]. Neural stem cells are commonly cultured in vitro as so called neurospheres – floating heterogenous aggregates of cells, containing a large proportion of stem cells.
-
Olfactory adult stem cells: Olfactory adult stem cells have been successfully harvested from the human olfactory mucosa cells, the lining of nose involved in the sense of smell [5].
-
Adipose derived adult stem cells: These cells have also been isolated from human fat, usually by method of liposuction. This cell population seems to be similar in many ways to mesenchymal stem cells derived from bone marrow. Human adipose derived stem cells (ASC’s) have been shown to differentiate in the lab into bone, cartilage, fat, muscle and might be able to differentiate into neurons, making them a possible source for future application in the clinic [24, 25].
-
Multipotent adult progenitor cells: The adult bone marrow also harbors a heterogeneous population of stem cells, which appear to have very broad developmental capabilities called multipotent adult progenitor cells. It has been proposed that multipotent adult progenitor cells constitute a population of stem cells derived from or closely related to embryonic stem cells [26] i.e. may be adult counterpart of embryonic stem cells.
PRESENT SCENARIO IN STEM CELL THERAPY [3]
Following types of stem cell therapy is possible in present scenario:
- Allogenic stem cell therapy: matched or unmatched
- Syngenic stem cell transplant: Identical twin
- Autologous stem cell transplant
- Cord blood stem cell transplant
- Nonmyeloablative stem cell transplant
However stem cell therapy has some inherent complications such as infection, regimen toxicity, carcinogenicity, immune deficiency and mortality due to co-occurrence of complications. These factors make the usage of stem cell limited. These factors not only alarm the treating team but also open new areas of research.
Clinical application and potential use of embryonic and adult stem cells [27]
There are many ways in which human stem cells can be used in basic research and in clinical research. These are:
-
Embryonic stem cells have been used to study the specific signals and differentiation steps required for the development of many tissues.
- Genetic therapy: Embryonic stem cells benefit the gene therapy by the following ways:
-
First human embryonic stem cells could be genetically manipulated to introduce the therapeutic gene. This gene may either be active or awaiting later activation, once the modified embryonic stem cells has differentiated into the desired cell type. Recently published reports establish the feasibility of such an approach [28]. Skin cells from an immunodeficient mouse were used to generate cellular therapy that partially restored function in the mouse. This can also be used in treating human patient with immuno deficiency.
-
Embryonic stem cells may additionally be indirectly beneficial for cellular gene therapy. Since these cells can be differentiated into many cell types, including presumably tissue specific stem cells, they may provide a constant in vitro source of cellular material. Such "adult" stem cells derived form embryonic stem cells may thus be utilized to optimize protocols for propagation and genetic manipulation technique [29].
-
-
Drug Testing: Because embryonic stem cells can proliferate without limit and can contribute to any cell type, human embryonic stem cells offer an unprecedented access to tissue from the human body. They will support basic research on the differentiation and function of human tissues and provide materials for testing that may improve the safety and efficacy of human drugs [30, 31] for example, new drugs are not generally tested on human heart cells because no human heart cell lines exist. Instead researchers rely on animal models. Because of important species specific differences between animal and human heart, however, drugs that are toxic to the human heart have occasionally entered clinical trials, sometimes resulting in death. Human ES cells – derived heart cells may be extremely valuable in identifying such drugs before they are used in clinical trials, there by accelerating the drug discovery process and leading to safer and more effective treatments [32-34].
-
Cell based therapies: It is perhaps the most important potential application of human stem cells. They generate cells and tissues that could be used for cells based therapies. Stem cells, directed to differentiate into specific cell types, offer the possibility of a renewable source of replacement cells and tissues to treat various disease.
-
Brain Damage: In the case of brain injury although reparative process appears to initiate, substantial recovery is rarely observed in adults suggesting a lack of robustness. Recently from research conducted in rats subjected to stroke suggested that administration of drugs to increase the stem cell division rate and direct the survival and differentiation of newly formed cells could be successful [8, 35, 36].
-
Cancer: Researcher at Harvard Medical School caused intracranial tumor in rodents. Then they injected human neural stem cells. Within days the cells had migrated into the cancerous and produced cytosine deaminase, an enzyme that convents a non-toxic pro-drug into a chemotherapeutic agent. As a result, the injected substance was able to reduce tumor mass by 80 percent [12, 21].
-
Spinal cord injury: Recently extensive study work is carried out in treating spinal cord injury. Scientist have treated the patient of spinal cord injury by isolating adult stem cells from umbilical cord blood and then injected them into damaged part of the spinal cord [37].
-
Muscle damage: Adult stem cells are also apparently able to repair muscle damaged after heart attacks. Heart attacks are due to coronary artery being blocked, staring tissue of oxygen and nutrients. Days after the attack is over, the cells try to remodel themselves in order to become able to pump harder. However, because of the decreased blood flow this attempt is futile and results in even more muscle cells dying. Researchers found that injecting bone marrow stem cells, a form of adult stem cells, into mice which had heart attacks induced resulted in an improvement of 33% in the functioning of heart. The damaged tissue had regrown by 68% [14, 38].
-
Heart damage: Several clinical trials targeting heart disease have shown that adult stem cell therapy is safe. However none of these trials have proven efficacy. Recently the use of patients own bone marrow derived stem cells and peripheral blood derived stem cells is becoming popular [33, 34].
CONTROVERSIES IN STEM CELL RESEARCH
Stem cell research is a minefield of ethical problems because stem cells that offer the most potential for study must be harvested from human embryos that are a few days old. In 1996, the birth of Dolly the sheep -- the world’s first successfully cloned mammal -- ignited a firestorm of protest and concern. The most famous controversy in stem cell research has been Hwang’s claim of cloning a dog. Hwang's work was able to offer an alternative to use of actual human embryo by cloning several human embryos, helping to eliminate the need for new embryos. Hwang claimed he had successfully cloned 30 human embryos, claims that have now been shown to be lies. Unfortunately, the use and study of embryonic stem cells are currently clouded by ethical controversy. Adult stem cells offer a unique alternative in that they may be isolated, studied, or manipulated without harming the donor. Currently, several obstacles for use of adult stem cells as therapy exist. First, the ability to identify most adult stem cells is impeded by lack of stem cell markers. Second, in vitro systems for manipulating adult stem cell populations are often not well defined. Finally, our understanding of how adult stem cells are regulated within their niche is in its infancy.
FUTURE PERSPECTIVES OF STEM CELL RESEARCH
-
Low blood supply: Now the method to produce large numbers of Red blood cells has been developed. In this method precursor Red blood cells, called hematopoietic stem cells are grown together with stromal cells, creating an environment that mimic the conditions of bone marrow, the natural site of red blood cell growth. Erythropoietin, a growth factor, is added coaxing the stem cells to complete terminal differentiation to red blood cells.
- Further research into this technique will have potential benefits to gene therapy and blood transfusion.
-
Baldness: Hair follicles also contain stem cells, and some researchers predict research on these follicle. Stem cell may lead to successes in treating baldness through "hair multi-placation" and known as "hair cloning" as early 2011. This treatment is expected to work through taking stem cells from existing follicles, multiplying them in cultures, and implanting the new follicle cells which have shrunk during the ageing process, which in turn respond to these signals by regenerating and once again making healthy air [17].
-
Missing teeth: The work on tooth generation has reached to a stage that it will be available to the general population in that decade. In theory, stem cells taken from the patient could be coaxed in the lab into turning into a tooth bud which, when implanted in the gums, will give rise to a new tooth, which would be expected to take two months to grow. It will fuse with jaw bones and release chemicals that encourage nerve and blood vessels to connect with it.
- Deafness: Those have been success in regrowing cochlear hair cells with the use of stem cells.
-
Blindness and vision improvement: Since 2003 research have successfully transplanted retinal stem cells into damaged eye to restore vision. Using embryonic stem cells, scientists become able to grow the sheet of top potent stem cells in the laboratory. When these sheets are transplanted over the damaged retina, the stem cells stimulate neural repair, eventually restoring vision. The group led by Dr. Sheraz Daya was able to successfully use adult stem cells obtained from the patient, a relative, or even a cadaver. Further rounds of trials are ongoing. [18]
-
Bone regenerations: Mesenchymal stem cells can be pumped and cutters expanded from animals and human and have been shown to regenerate functional tissue when delivered to the site of musculo-skeletal defects in experimental animals. Mesenchymal stem cells can regenerate bone in a clinically significant osseous defect and may therefore provide an alternative to autogenous bone grafts.
-
Diabetes Type I: In people who suffer from type I diabetes, the cells of the pancreas that normally produce insulin are destroyed by the patient's own immune system. New studies indicate that it may be possible to direct the differentiation of human embryonic stem cells in the cell culture to form insulin-producing cells that eventually could be used in transplantation therapy for diabetics.
ETHICAL CONCERNS IN STEM CELL RESEARCH
In the case of embryonic stem cell research, the end that scientists hope to achieve is the relief of human suffering. That this is a humanitarian and worthy end is not in dispute. The controversy is about the means, namely, the consumption of donated embryos. More particularly, embryonic stem cell research and therapy would use donated embryos that, by virtue of donor instructions, will never enter a uterus. Is it permissible to use those means to that end? Our task is to decide how we should act toward an embryo, and whether we should recognize, as we do among adults, distinctions between embryos of various types and in various circumstances. We immediately encounter the question of what beings we should classify as "persons" for purposes of the duty not to kill persons. For one who concludes that we are not obliged to refrain from using embryos that will never enter a womb, embryonic stem cell research is a case of fostering a worthy end by using only nonpersons as means.
CONCLUSION
Stem cells pose a bright future for the therapeutic world by promising treatment options for the diseases which are considered as noncurable now a days. However, because of significant peri and post-transplant morbidity and mortality further research and trials are required to refine and optimize conditioning regimens and modalities of supportive care. By virtue of funding of stem cell research, we hope to see new horizon of therapeutics in the form of organ development and replacement of lost tissue such as hairs, tooth, retina and cochlear cells.
REFERENCES
- Becker AJ, McCulloch EA, Till JE. Cytological demonstration of the clonal nature of spleen colonies derived from transplanted mouse marrow cells. Nature. 1963;197:452-4.
- Siminovitch L, McCulloch EA, Till JE. The distribution of colony-forming cells among spleen colonies. J Cell Physiol. 1963 Dec;62:327-36.
- Velu Nair. Stem cell transplantation. API medical update. 2004;14:366-77.
- Friedenstein AJ, Gorskaja JF, Kulagina NN. Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp Hemato. 1976 Sep;l 4(5):267-74.
- Murrell W, Feron F, Wetzig A, el al. Multipotent stem cells from adult olfactory mucosa. Dev Dyn. 2005 Jun;233(2):496-515.
- Niwa H, Miyazaki J, Smith AG. Quantitative expression of Oct-3/4 defines differentiation, dedifferentiation or self-renewal of ES cells. Nat Genet. 2000 Apr;24(4):372-6.
- Caveleri F, Scholar HR. Nanog: a new recruit to embryonic stem cell orchestra. Cell. 2003 May;113:551-2.
- Wang X, Yang YJ, Jia YJ, et al. The best site of transplantation of neural stem cells into brain in treatment of hypoxic-ishemic damage: experiment with newborn rats. Zhonghua Yi Xue Za Zhi. 2007 Mar 27;87(12):847-50.
- Molofsky AV, Pardal R, Iwashita T, el al. Bmi-1 dependence distinguishes neural stem cell self-renewal from progenitor proliferation. Nature. 2003 Oct 30;425 (6961):962-7.
- Park IK, Qian D, Kiel M, el al. Bmi-1 is required for maintenance of adult self-renewing haematopoietic stem cells. Nature. 2003 May 15;423(6967):302-5.
- Dontu G, Jackson KW, McNicholas E, el al. Role of Notch signaling in cell-fate determination of human mammary stem/progenitor cells. Breast Cancer Res. 2004;6(6):R605-15.
- Beachy PA, Karhadkar SS, Berman DM. Tissue repair and stem cell renewal in carcinogenesis. Nature. 2004 Nov 18;432(7015):324-31.
- Rosenthal N. Prometheus’s vulture and the stem-cell promise. N Engl J Med. 2003 Jul 17;349(3):267-74.
- Korbling M, Estrove Z. Adult stem cells for tissue repair-a new therapeutic concept? N Engl J Med. 2003 Aug 7;349(6):570-82.
- Marshall GP 2nd, Laywell ED, Zheng T, et al. In vitro-derived "neural stem cells" function as neural progenitors without the capacity for self-renewal. Stem Cells. 2006 Mar;24 (3):731-8.
- Lavker RM, Sun TT. Epidermal Stem cells: properties, markers, and location. Proc Natl Acad Sci USA. 2000 Dec 5;97(25):13473-5.
- Alonso L, Fuchs E. Stem cells in the skin: Waste not, Wnt not. Genes Dev. 2003 May 15;17(10):1189-200.
- Tsceng SCG, Sun TT. Stem cells: Ocular surface maintenance. In Brightbill FS (ed): Corneal surgery: Theory, techniques and tissue, 3rd ed. New York, Mosby,1999:9-18.
- Verfaillie CM. Hematopoietic stem cells for transplantation. Nat Immunol. 2002 Apr;3(4):314-7.
- Orkin SH, Morrison SJ. Stem-cell competition. Nature. 2002 Jul 4;418(6893):25-7.
- Liu S, Dontu G, Wicha MS. Mammary stem cells, self-renewal pathways, and carcinogenesis. Breast Cancer Res. 2005;7(3):86-95.
- Shackleton M, Vaillant F, Simpson KJ, et al. Generation of a functional mammary gland from a single stem cell. Nature. 2006;439:84-8.
- Bull ND, Bartlett PF. The adult mouse hippocampal progenitor is neurogenic but not a stem cell. J Neurosci. 2005 Nov 23;25(47):10815-21.
- Zuk PA, Zhu M, Mizuno H, et al. Mutilineage cells derived from human adipose tissue: a putative source of stem cells for tissue engineering. Tissue Engineering. 2001;7(2):211-6.
- Zuk PA, Zhu M, Ashjian P, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002 Dec;13(12):4279-95.
- Jiang Y, Vaessen B, Lenvik T, et al. Multipotent progenitor cells can be isolated from postnatal murine bone marrow, muscle and brain. Exp Hematol. 2002 Aug;30(8):896-904.
- Tuch BE. Stem cells--a clinical update. Aust Fam Physician. 2006 Sep;35(9):719-21.
- Rideout WM 3rd, Hochedlinger K, Kyba M, et al. Correction of a genetic defect by nuclear transplantation and combined cell and gene therapy. Cell. 2002 Apr 5;109(1):17-27.
- Mitsui K, Tokuzawa Y, Itoh H, et al. The homeoprotein Nanog is required for maintenance of pluripotency in mouse epiblast and ES cells. Cell. 2003 May 30;113(5):631-42.
- Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981 Jul 9;292(5819):154-6.
- Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci USA. 1981 Dec;78(12):7634-8.
- He JQ, Ma Y, Lee Y, et al. Human embryonic stem cells develop into multiple types of cardiac myocytes: action potential characterization. Circ Res. 2003 Jul 11;93(1):32-9.
- Mummery C, Ward-van Oostwaard D, Doevendans P, et al. Differentiation of human embryonic stem cells to cardiomyocytes: role of coculture with visceral endoderm-like cells. Circulation. 2003;107:2733-40.
- Vanderlaan RD, Oudit GY, Backx PH. Electrophysiological profiling of cardiomyocytes in embryonic bodies derived from human embryonic stem cells. Circ Res. 2003 Jul 11;93(1):1-3.
- Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992 Mar 27;255(5052):1707-10.
- Vawda R, Woodbury J, Covey M, et al. Stem cell therapies for perinatal brain injuries. Semin Fetal Neonatal Med. 2007 Aug;12(4):259-72.
- Rolletschek A, Blyszczuk P, Wobus AM. Embryonic stem cell-derived cardiac, neuronal and pancreatic cells as model systems to study toxicological effects. Toxicol Lett. 2004 Apr 1;149(1-3):361-9.
- Patrick C H Hsieh, Vincent F M Segers, Michael E Davis, et al. Evidence from a genetic fate-mapping study that stem cells refresh adult mammalian cardiomyocytes after injury. Nature Medicine. 2007;13:970-4.