INTRODUCTION
The debilitating mosquito-borne viral disease Chikungunya has been mapped as a major public and international health concern following minor epidemic outbreaks from January to April 2005 and severe epidemics from January to April 2006 in the Indian Ocean islands of Reunion, Mauritius, Comoros and Mayotte. [1] India was also severely affected. [2, 3] A number of imported cases originating from these countries have been registered in Europe and North America. [4, 5] The epidemics seem to have originated in the Kenyan coastal towns of Lamu (June 2004) and Mombasa (November 2004) where unsafe water storage and elevated temperatures during an unusually dry climatic period may have facilitated the development of mosquitoes and transmission of the Chikungunya virus. [6] The disease then spread to the Comoros, Reunion, and Mayotte before reaching Mauritius in April 2005. [1] In Reunion and Mauritius, the pace of the viral spread slowed during the cooler months of June to October, picking up explosively in January 2006 in Reunion and in early February 2006 in Mauritius. The epidemic peaked in March 2006 in these two islands, with most cases appearing in the months of February and March, and died out towards the end of April 2006 in Mauritius. [7] The disease had in the meantime re-emerged in India after an absence of 23 years [2, 3] before affecting the Maldives [8]. More than a million people have been affected by Chikungunya since January 2005. [9] There have been recent outbreaks in the West African state of Gabon [10] and in the Ravenna province in northern Italy [11].
The Chikungunya virus (CHIKV), an alphavirus of the family Togaviridae [12], is believed to have originated in Africa [13] where it was first isolated in Tanzania in 1953 [14]. It appears in three distinct genotypes: the West African, Central/East African, and Asian genotypes. [15] In Africa, CHIKV is maintained in a sylvan reservoir involving wild primates and forest-dwelling aedes spp. mosquitoes. [13] It is believed that, in Asia, the virus is maintained by a human-mosquito (aedes aegypti) urban cycle. [3] The disease itself is characterized by an incubation period of 4-5 days followed by fever, skin rash, joint swelling, and a debilitating, sometimes recurrent, arthralgia. [12, 16] The arthralgia is a prominent feature of Chikungunya and helps to clinically distinguish it from dengue, a mosquito-borne viral disease for which Chikungunya is often mistaken because of the similarity of symptoms. [3] It is thought that CHIKV infection confers life-long immunity to the disease. [12] There is as yet no evidence for the vertical transmission of the virus in mosquitoes [17] although vertical maternal fetal transmission has been reported in Reunion Island [18].
Although aedes aegypti has so for long been considered to be the primary mosquito vector of CHIKV, aedes albopictus has been the incriminated vector during the recent epidemics in Reunion and Mauritius. [15] These vectors are peridomestic, anthropophilic, and diurnal. However, whereas aedes aegypti is endophilic, aedes albopictus is exophilic. Aedes albopictus is also an aggressive opportunistic biter, preferring its blood meals early morning and late afternoons. [19] The endophilic nature of aedes aegypti led to its near-eradication in Reunion and eradication in Mauritius during the successful DDT indoor wall-spraying campaign against anopheles gambiae and malaria in the late 1940s/ early 1950s. [20] About one third of a population of 775000 people has been affected by Chikungunya in Reunion. [9] According to official figures, the number of Mauritians affected in the 2006-outbreak was nearly 11000 but, extrapolating from the fact that 32 of the 21000 tourists who visited Mauritius from the United Kingdom from February 2006 to April 2006 caught the disease [21], this number is likely to have been higher. It is speculated that the scale of the outbreaks was due to the virus (Central/East African genotype) entering immunologically naive populations and also possibly to a genome microevolution of the virus leading to an increased adaptability with aedes albopictus. [15]
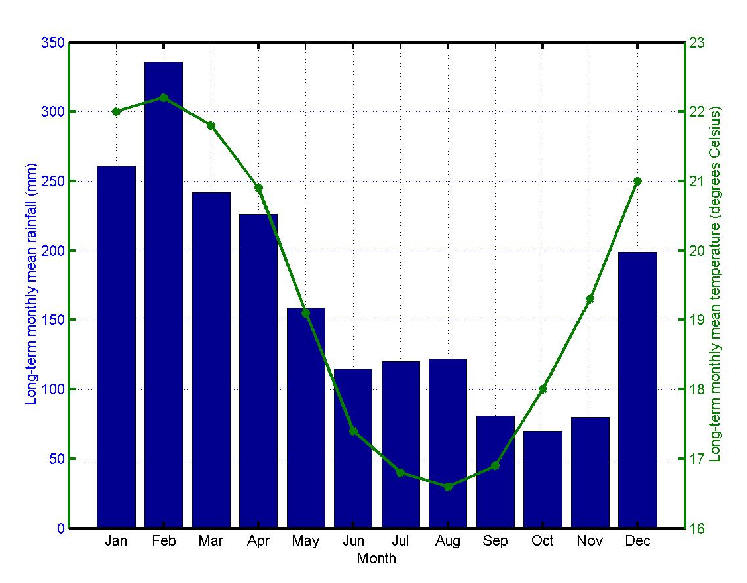
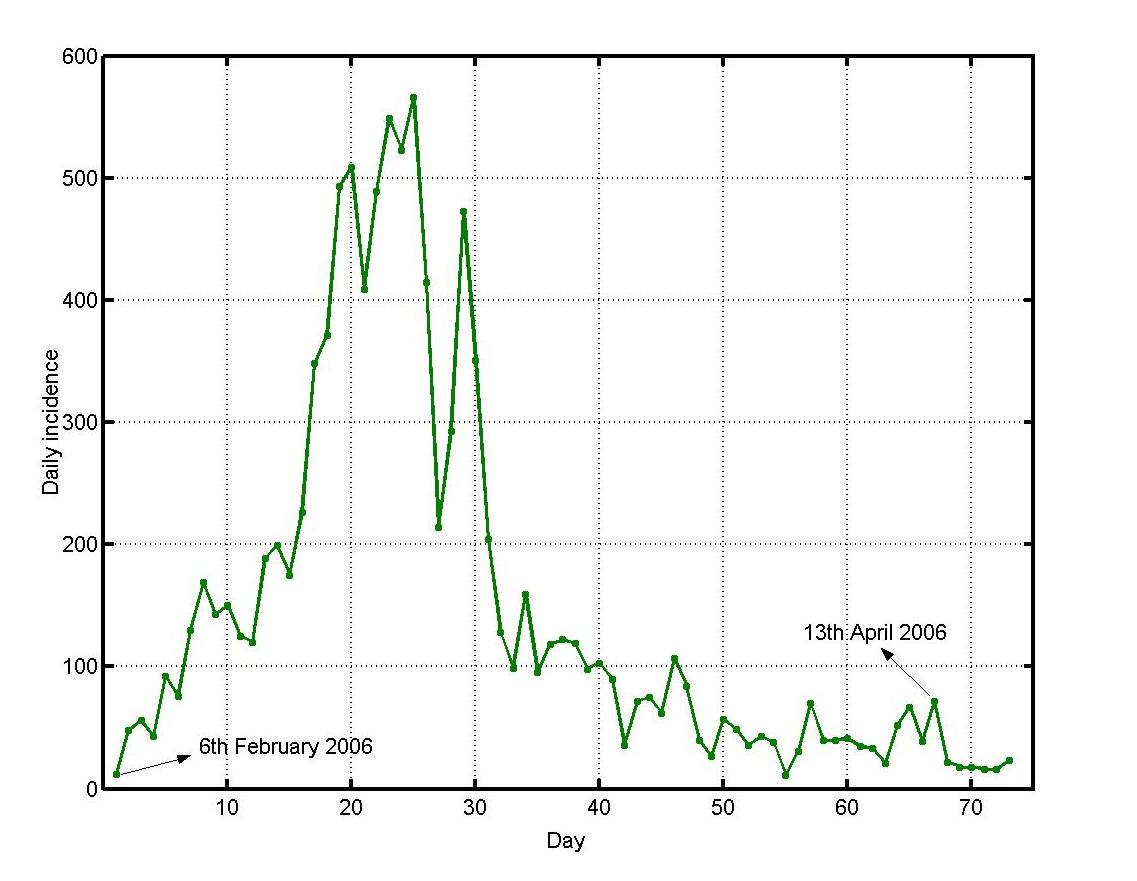
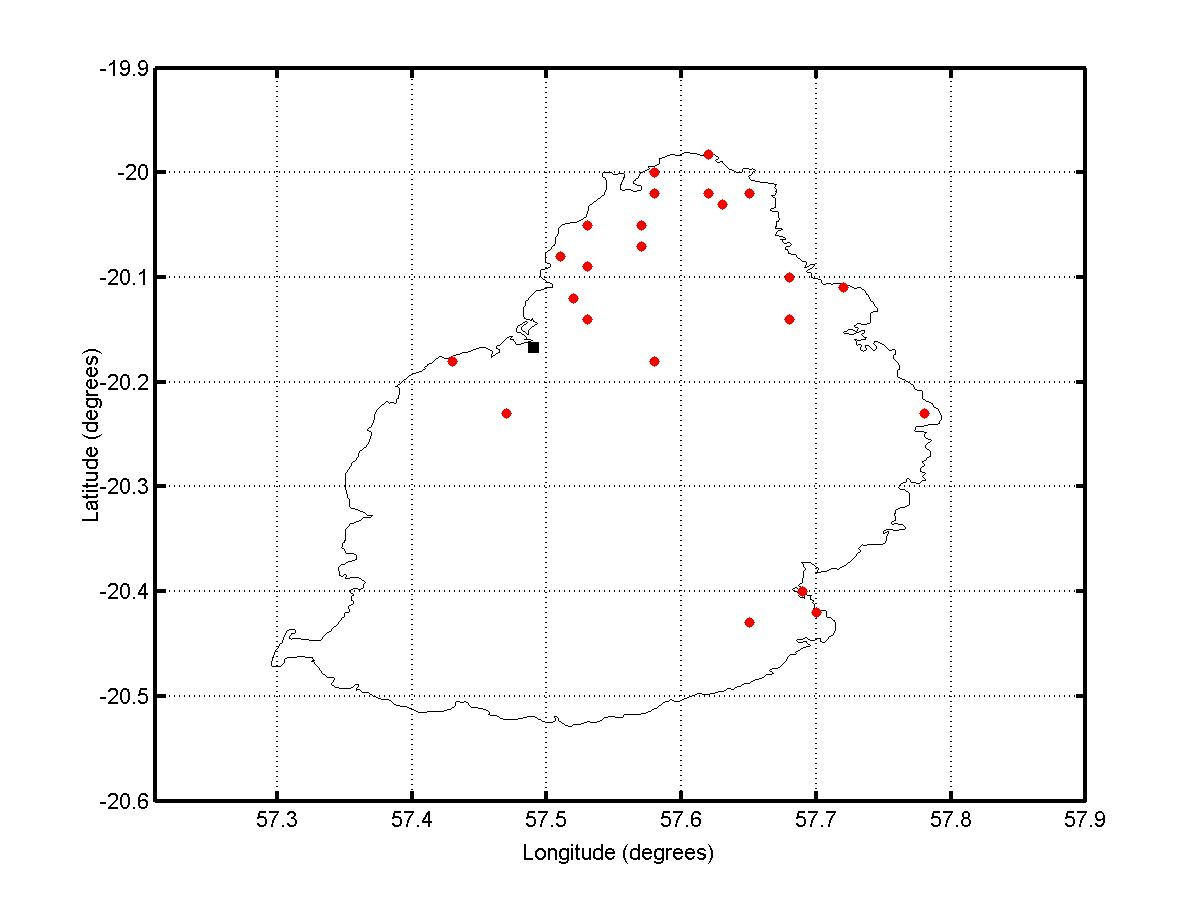
Mauritius (population 1.2 million/ 1865 sq. km) is a small island developing state situated between latitudes 19.98° and 20.53°S and longitudes 57.30° and 57.79°E. About 20% of its surface consists of built-up areas. The remaining 80% comprises agricultural land, forest land and other uninhabited land. The main residential regions have population densities ranging from 1500-8000 per sq. km. [22] Summer (November to April) is accompanied by heavy rainfall especially from December to March. The major Chikungunya epidemic outbreak of February/March 2006 [1] had been preceded by a minor one in April/May 2005 which was localized in Port Louis, the capital city, [23] and is believed to have originated in a hostel of the city often used by visitors from the neighboring Comoros [24] where Chikungunya had been reported in January 2005 [25]. The number of Mauritians affected during the 2005 outbreak was nearly 3600. It is believed that, during the cooler and drier months of May to October 2005 (Figure 1), the virus spread slowly to the north of Mauritius. The small number of cases during that time was interpreted as the end of the epidemic. The island, however, suffered from an explosive epidemic in February/March 2006. Figure 2 shows the daily incidence of Chikungunya in Mauritius during that period. The north and south-east of the island were the most severely affected regions as shown in Figure 3.
The objectives of this study were to understand the timing and development of the 2006-outbreak in Mauritius, to investigate the possibility of a future outbreak, and to propose measures to prevent the recurrence of an epidemic in Mauritius.
 |
 |
 |
METHODOLOGY
Meteorological data analysis
Mauritius rainfall, temperature and humidity monthly means for the period January 2005 to December 2006 were collected from the Mauritius Meteorological Services and compared with their 1971-2000 long-term monthly means.
Survey
The surveyed locality was Triolet (20.05°S, 57.53°E) in the north-west of the island. Triolet has a human population of 22808 and an area of 13.06 sq. km. The survey was a door-to-door household census-type survey. It was conducted in a 0.6 sq. km area in the centre of the locality. The surveyed area was divided into sub-areas of size 0.2Km x 0.2Km. In each household, at least one responsible adult was interviewed. All interviews were carried out by the same person to ensure the consistency of the data collected. A suspected Chikungunya affected person was identified as one who had been clinically diagnosed as so. If a household was unoccupied during the first visit, there was a second visit to ensure completeness of data collection. The survey was carried out over the two months period of November and December 2006. The survey sought the following information:
- the number of persons in each household,
- the number of persons in those households who had been diagnosed as having Chikungunya,
- where the affected persons thought they had acquired the infection,
- details about the precautions they have been taking against Chikungunya since the epidemic, and
- the risk factors associated with Chikungunya propagation in each survey sub-area.
Mathematical modeling
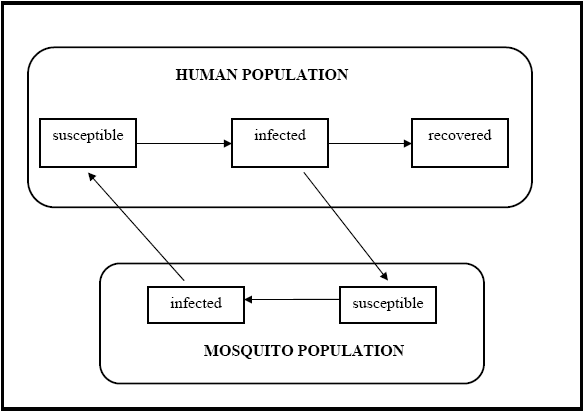
A compartmental human-mosquito interaction model [26] was used to simulate the temporal evolution of Chikungunya in a locality. A schematic diagram of the model is shown in Figure 4.
 |
Denoting the human and mosquito population sizes by Nh and Nv respectively, the number of susceptible humans by Sh, and the number of infected humans and mosquitoes by Ih and Iv respectively, the following differential equations describe the time evolution of Sh, Ih and Iv:
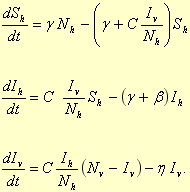 |
Infected humans and infected mosquitoes were assumed to be infectious. The lifetime of a human was 1/γ, that of a mosquito was 1/η, and the human infectious period was 1/β. It was assumed that infected mosquitoes and susceptible mosquitoes had the same biting rate and that the probability of the virus transmission from an infected mosquito to a susceptible human during a bite was the same as that from an infected human to a susceptible mosquito. The product of the mosquito biting rate and the probability of the transmission of the virus was denoted by C. It was further assumed that aedes albopictus mosquitoes had a flight range of one kilometer [27] and that, because of the random mixing assumption of the model, the populations Nh and Nv represented population densities per sq. km.
The model was integrated to compute the evolution of the outbreak for a period of 60 days in a theoretical locality with a human population of density 3000 per sq. km and with initially one infected human but an otherwise susceptible human and mosquito population. The lifetimes of humans and mosquitoes were respectively taken to be 70 years and 30 days. During the outbreak it was assumed that the human population was constant and that the mosquito population had attained its carrying capacity during that time and was therefore constant and that both these populations were homogeneously spatially distributed over the locality. Further assumptions included the following: the mosquito biting rate was once weekly, the probability of the virus transmission during a bite was 0.9, the human infectious period was 3 days and the mosquito population was four times greater than the human population.
The behavior of a follow-up outbreak in the theoretical locality was investigated by computing the evolution of the disease with the number of humans affected in the previous outbreaks as having acquired immunity, but with otherwise the same initial conditions. A disease-acquired herd immunity level for this locality was deduced from the results of computations with different levels of initial acquired immunity.
First-outbreak computations were performed for a locality of population density of 7000 per sq. km such as the centre of Triolet for different initial small numbers of infected humans and mosquitoes.
In a mosquito-control scenario for the theoretical locality, the evolution of the epidemic was computed with the number of infected adult mosquitoes controlled to one per sq. km every seven days.
DISCUSSION
Meteorological data analysis
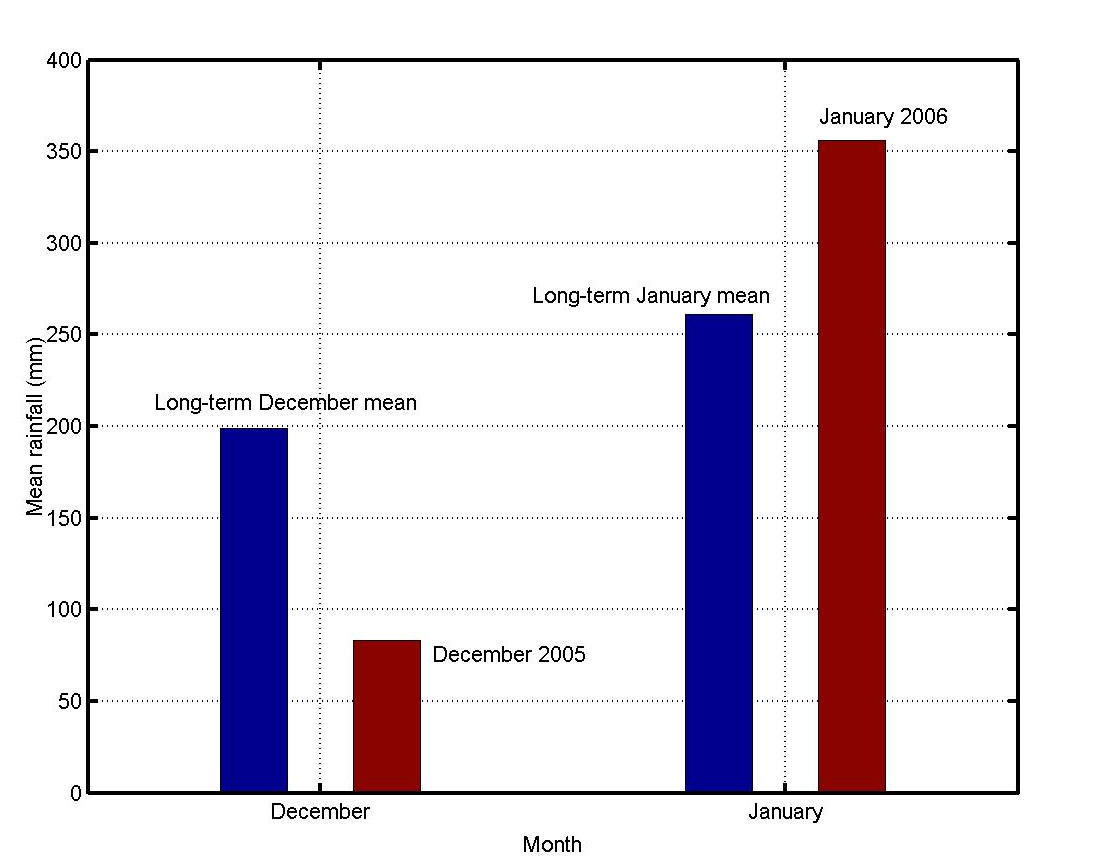
December 2005 was a relatively dry month. The mean monthly rainfall over Mauritius for this month was 42% of the 1971-2000 long term mean. The first three weeks of January 2006 were also relatively dry. However as from the 25th January, Mauritius was under the influence of meteorological system which caused heavy precipitation. By the end of the month, the mean monthly rainfall over Mauritius was 36% above the long-term mean (Figure 5). For December 2005, the mean monthly maximum temperature was higher than the long-term mean by 0.2 ºC. For January to March 2006, the mean monthly maximum temperature was slightly lower than the long term mean. However, the mean monthly minimum was higher than the long-term mean for both years 2005 and 2006. The maximum deviation for the period December to March 2006 was in March 2006 when the mean monthly minimum temperature was higher than the long-term mean by 1.2 ºC. The humidity for December 2005, January 2006, February 2006 and March 2006 was higher than the long-term mean by 2.6%, 4.6%, 2.2% and 5.6% respectively.
 |
Survey
The survey covered 691 households (3378 inhabitants) out of an estimated total of 750 households and touched at least 75% of the population in each of the 0.2Km × 0.2Km sub-area. 51% of the population surveyed were suspected to have been affected by the 2006-epidemic. The percentage of suspected affected cases in the sub-areas ranged from a minimum of 44.7% to a maximum of 70.3%. In 10 of the 15 sub-areas, this percentage exceeded 50%. Most of the suspected cases thought they had been infected inside or in the immediate vicinity of their houses. Risk factors associated with Chikungunya propagation were found to be mainly houses with still water on their flat roofs, unattended bushy areas and shady areas surrounding the house. These risk factors were found to have been homogeneously distributed over the surveyed area at the time of the epidemic.
Mathematical modeling
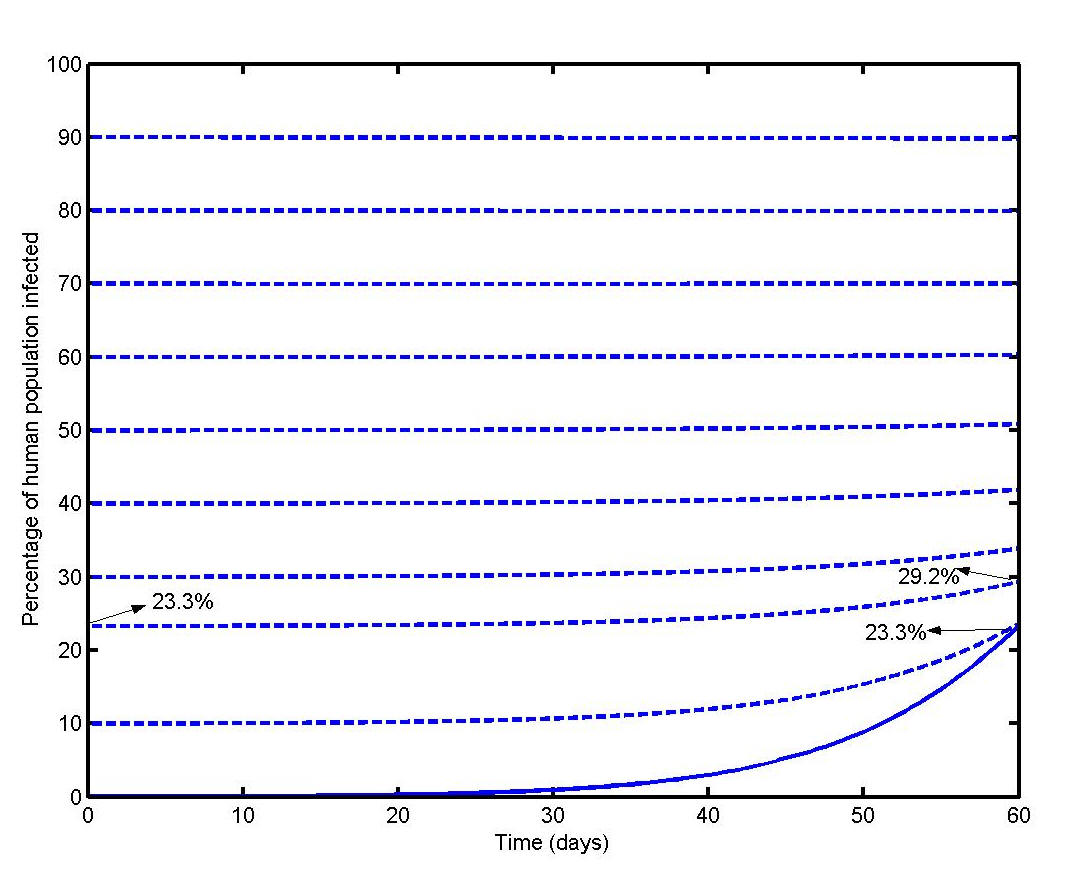
The evolution of an outbreak in the theoretical locality is shown in Figure 6 (full line). 23.3% of the population was forecasted to have been infected at the end of 60 days corresponding to the months of February and March 2006. In a follow-up outbreak, with a starting point of 23.3% of the population having acquired immunity (as a result of previous outbreaks), the percentage of infected population at the end of 60 days was found to be 29.2%, i.e. an additional 5.9%. It is also apparent from Figure 6 that herd immunity would have been reached when about 60% of the population had been initially infected. For a first outbreak in a locality with a population density of 7000 per sq. km, such as the centre of Triolet, the computed percentage of infected people at the end of 60 days with initially one infected human and no infected mosquitoes was 11.6%. When the computation was carried out with initially 4 infected humans and 5 infected adult mosquitoes, the result was 51.7%.
 |
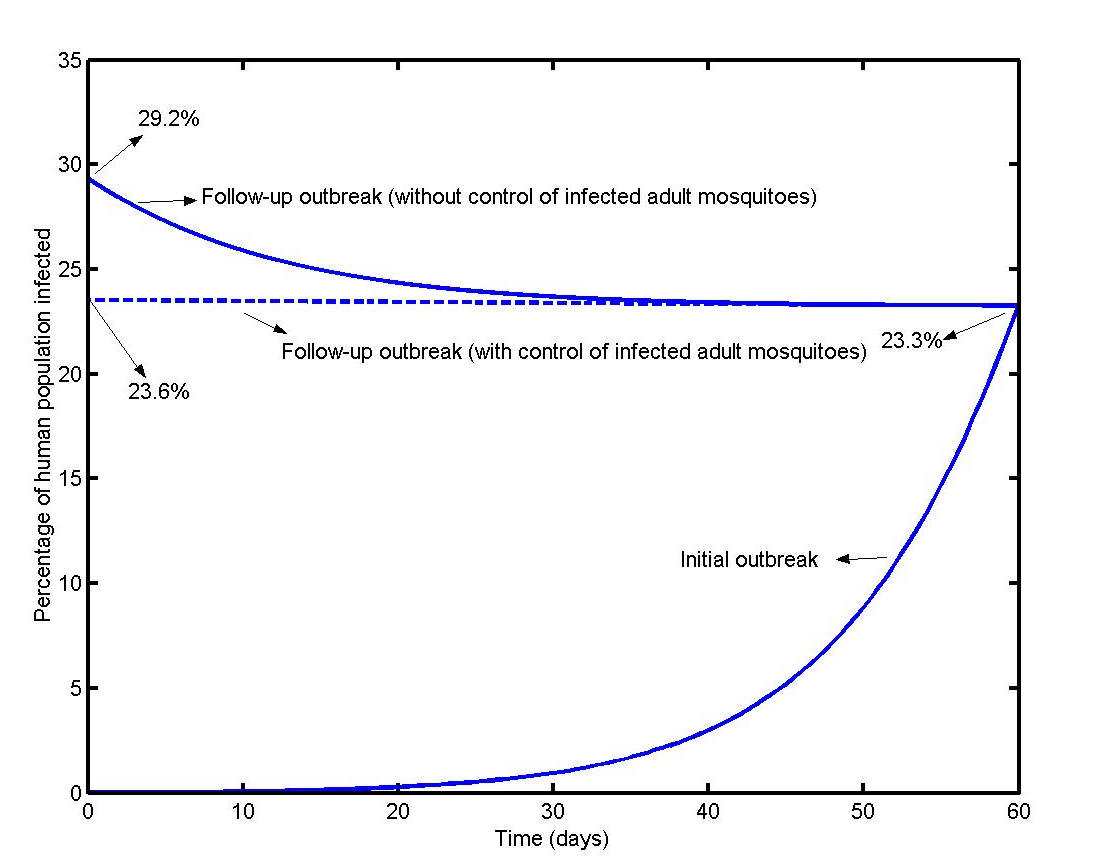
The time evolution of a follow-up outbreak in the theoretical locality with and without the control of infected adult mosquitoes assuming that 23.3% of the human population had acquired immunity as a result of previous outbreaks is illustrated in Figure 7. In the infected adult mosquito control scenario, only an additional 0.3% of the population was computed to have been infected at the end of 60 days whereas if there had been no control the additional percentage of people infected was 5.9%.
 |
DISCUSSION
This study integrated meteorological data analysis, a population survey in a study locality and mathematical modeling to provide a picture of Chikungunya evolution in Mauritius during the period February/March 2006. The Mauritius Meteorological Services data have a high index of reliability. During the household survey, care was taken to minimize missing data and to ensure a high response rate. In addition the interviewer also carried out a visual inspection to confirm prevailing risk factors in the locality. Limitations of the survey included the recall bias of the interviewed inhabitants and clinical diagnosis not supported by serological evidence. The modeling was itself limited by the random mixing assumption.
It is well-known that a habit of aedes albopictus mosquitoes is to their lay their eggs in dry areas in anticipation of heavy rainfall. The abnormally high rainfall in the third week of January 2006 witnessed a sharp increase in the aedes albopictus population 1-2 weeks later and this preceded the onset of the explosive epidemic of February/March 2006. In Mauritius, there were about 777 medically qualified practitioners in the public sector and 565 in the private sector at that time. [28] Many Chikungunya cases were then treated by medically qualified practitioners from the private sector and, in the absence of a formal surveillance system, a proportion of these cases may not have been registered. Regarding the surveyed locality, computations support the view that during the cooler and drier months of May to October 2005, the virus had been spreading slowly in the north of Mauritius and that there were a few infected individuals and mosquitoes present in the locality prior to the 2006 epidemic, sufficient enough to trigger a large outbreak.
Our computations also suggest a disease-acquired herd immunity threshold of about 60% in the theoretical locality as propagation of the infection was minimal above this figure. We believe that herd immunity has almost been reached in this Triolet and in much of the north of Mauritius. On the other hand, the current level of acquired immunity in the theoretical locality and in similar localities of Mauritius would still be low enough to allow future outbreaks to occur unless precautionary measures are taken and sustained.
It is currently believed that, in the absence of a vaccine, mosquito control is the sole available method for reducing the transmission of the Chikungunya virus. [19] It has also been seen that traditional large scale campaigns against aedes albopictus may be ineffective. [19] One of the main conclusions from our computations is that it is possible to contain the propagation of Chikungunya infection by controlling the number of infected adult mosquitoes.
Assuming that there is no animal Chikungunya reservoir in Mauritius, we propose the following strategy to control the population of infected adult mosquitoes to a minimum and help prevent future outbreaks:
- Creation of a sentinel network to alert Mauritian health authorities as soon as a Chikungunya case is diagnosed or suspected.
- Implementation of case isolation and case protection measures when a case is diagnosed/ suspected.
- Implementation of case contact tracing [29] measures when a case is diagnosed/ suspected.
- Implementation of case reactive mosquito control measures in an area of radius one kilometer within 36 hours of the alert from outwards towards the place of residence of the suspected case to eliminate infected adult mosquitoes.
- Implementation of case reactive mosquito control measures in an area of radius one kilometer within immediate effect from outwards towards the place(s) where the suspected case may have caught the virus to eliminate infected adult mosquitoes.
The suggested time frames take into account the virus latent period (3-4 days in humans and 4-5 days in mosquitoes), its incubation period (4-5 days) and the infectious period in humans (3 days). The one-kilometer radius is derived from aedes albopictus dispersal studies [27] and remains be confirmed in Mauritius. The proposed case-reactive strategy needs to be complemented by pre-emptive measures such as the mosquito source reduction programs and public education campaigns already undertaken by Mauritian authorities. During our household surveys, it was noted that households had initiated measures to control mosquito breeding sites, e.g., by regularly draining accumulated rainwater from flat-roofed buildings and by adopting personal precautionary measures against mosquito bites. The pre-emptive measures have been successful so far but their full efficacy remains to be assessed considering that the disease may have entered a silent phase [30].
The 2005 and 2006 Chikungunya epidemics in Mauritius have highlighted the vulnerability of the island to infectious diseases. Being a prime tourist destination, Mauritius remains at risk to the entry and development of other serious mosquito-borne diseases such as dengue, which emerged in Reunion and Seychelles in the late 1970s [31] but has not as yet affected Mauritius and for which aedes albopictus can be a competent vector [19], and of other serious infectious diseases such as the avian flu [32]. Moreover, the 30000 macaques (Macaca fascicularis) present in the country’s national forests [33] and domestic animals could act as non-human reservoirs of the virus and contribute to the endemicity of CHIKV.
Dense international air traffic combined with factors such as the spread of aedes albopictus to north temperate countries [34], the ability of its eggs to tolerate freezing conditions [35], world rising temperatures and the adaptability of the East African strain of CHIKV to a vector originating from South East Asia [15] give a global dimension to the occurrence of Chikungunya in Mauritius and highlight an urgent need for local, regional and international sustained collaborative efforts to combat emerging and re-emerging viral infectious diseases in this region of the world.
CONCLUSION
The onset of the 2006 Chikungunya epidemic in Mauritius was rainfall driven. Simple mathematical models can provide valuable insight into epidemic-outbreak initial conditions and development. Localities of Mauritius where herd immunity have not been reached may experience an epidemic recurrence in the future. The case-reactive control of infected adult mosquitoes can be of primary importance in preventing epidemic outbreaks and recurrence.
ACKNOWLEDGEMENT
We are grateful to the Mauritius Meteorological Services for the release of meteorological data and thank P Reiter, J-P Chretien and C Comiskey for their comments on an initial version of the manuscript. Dr. S K Ramchurn acknowledges discussions with A Bheecaree, N Pyndiah and C Ragavoodoo, of the Ministry of Health and Quality of Life, Mauritius.
REFERENCES
- WHO Epidemic and Pandemic Alert and Response (EPR). Chikungunya in La Réunion (France), Mayotte, Maurice, Seychelles and India. Available: http://www.who.int/csr/don/2006_03_17/en/ accessed 30 May 2007.
- Kalantri SP, Joshi R, Riley LW. Chikungunya epidemic: An Indian perspective. Natl Med J India. 2006;19:315-22.
- Lahariya C, Pradhan SK. Emergence of chikungunya virus in Indian subcontinent after 32 years: a review. J Vec Borne Dis. 2006;43:151-60.
- Editorial team, Pfeffer M, Loescher T. Cases of chikungunya imported into Europe. Eurosurveillance 2006;11:060316. Available: http ://www.eurosurveillance.org/ew/2006/060316.asp#2
- Lanciotti RS, Kosoy OL, Laven JJ, et al. Chikungunya Virus in US Travelers Returning from India. Emerg Inf Dis. 2006;13:764-7.
- Chretien J-P, Anyamba A, Bedno SA, et al. Drought-associated Chikungunya emergence along coastal East Africa. Am J Trop Med Hyg. 2007;76:405-7.
- Chateau T. Chikungunya : Plan d’action pour l’hiver. L’Express Vendredi 5 Mai 2006. Available: http://www.lexpress.mu accessed 30 May 2007.
- Chikungunya – Indian Ocean Update (33): Maldives. proMED-mail 23 Dec 2006 20061224.3598 Available: http://www.promedmail.org accessed 30 May 2007.
- Pialoux G, Gaüzère BA, Jauréguiberry S, et al. Chikungunya, an epidemic arbovirosis. Lancet Infect Dis. 2007;7:319-27.
- Chikungunya – Gabon proMED-mail 11 May 2007 20070519.1591 Available: http://www.promedmail.org accessed 30 May 2007.
- Chikungunya – Italy (Emilia Romagna) proMED-mail 3 Sep 2007 20070903.2899 Available: http://www.promedmail.org accessed 6 Sep 2007.
- CDC. Chikungunya Fever Fact Sheet. Available: http ://www.cdc.gov/NCIDOD/DVBID/Chikungunya/chikvfact.htm
- Vanlandingham DL, Hong C, Klingler K, et al. Differential infectivities of O’nyong-nyong and Chikungunya virus isolates in Anopheles Gambiae and Aedes aegypti mosquitoes. Am J Trop Med Hyg. 2005;72:616-21.
- Ross RW. The Newala epidemic. III. The virus: isolation, pathogenic properties and relationship to the epidemic. J Hyg. 1956;54:177-91.
- Schuffeneker I, Iteman S, Michault A, et al. Genome microevolution of Chikungunya viruses causing the Indian Ocean outbreak. PLOS Med. 2006;3:1058-70.
- Ozden S, Huerre M, Riviere JP, et al. Human muscle satellite cells as targets of Chikungunya virus infection. PloS ONE. 2007;6:e527.
- Hundekar SL, Thakare JP, Gokhale MD, et al. Development of monoclonal antibody based antigen capture ELISA to detect chikungunya virus antigen in mosquitoes. Indian J Med Res. 2002;115:144-8.
- Robillard PY, Boumahni B, Gerardin P, et al. Vertical maternal fetal transmission of the Chikungunya virus. Ten cases among 84 pregnant women. Presse Med. 2006;35:785-8.
- Reiter P, Fontenille D, Paupy C. Aedes albopictus as an epidemic vector of chikungunya virus: another emerging problem? Lancet Infect Dis. 2006;6:463-4.
- Zeller HG. Dengue, arbovirus et migrations dans l’Océan Indien. Bull Soc Pathol Exo. 1998;91:55-60.
- Chikungunya: increase in imported cases. CDR Weekly 2006;16(21). Available: http://www.hpa.org.uk/cdr/archives/2006/cdr2106.pdf
- Population density per sq. km by Municipal Council Areas and Village Council Areas - Census 2000. Available: http://www.gov.mu/portal/sites/ncb/cso/report/hpcen00/census4/map23.htm accessed 30 May 2007.
- Chikungunya – Mauritius and Reunion island proMED-mail 19 May 2005 20050519.1372 Available: http://www.promedmail.org accessed 8 September 2006.
- Essackjee KD, Goorah SSD, Cheeneebash J. A clinical profile of the 2005 Chikungunya outbreak in Mauritius. In preparation.
- Chikungunya – Comoros proMED-mail 5 Apr 2005 20050405.0986 Available: http://www.promedmail.org accessed 8 September 2006.
- Derouich M, Boutayeb A, Twizell EH. A model of dengue fever. Biomed Eng. 2003;2:1.
- Honório NA, da Costa Silva W, Leite PJ, et al. Dispersal of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) in an Urban Endemic Dengue Area in the State of Rio de Janeiro. Brazil Mem Inst Oswaldo Cruz. 2003;98:191-8.
- Selected manpower statistics as at end of year 1996-2005, Republic of Mauritius. Available: http://www.gov.mu/portal/goc/moh/file/statsm05/infra05m/infra2.pdf
- Eames KT, Keeling MJ. Contact tracing and disease control. Proc Biol Sci. 2003;270:2565-71.
- Sam I-C, Abu Bakar S. Chikungunya virus infection. Med J Malaysia. 2006;61:264-9.
- Paupy C, Girod R, Salvan M, et al. Population structure of Aedes albopictus from La Réunion Island (Indian Ocean) with respect to susceptibility to a dengue virus. Heredity. 2001;87:273-83.
- WHO Epidemic and Pandemic Alert and Response (EPR). Avian influenza. Available: http://www.who.int/csr/disease/avian_influenza/en/ accessed 30 May 2007.
- Chastel C. Chikungunya virus: its recent spread to the southern Indian Ocean and Reunion Islands (2005-2006). Bull Acad Natl Med. 2005;89:1827-35.
- Aranda C, Eritja R, Roiz D. First record and establishment of the mosquito Aedes albopictus in Spain. Med Vet Entomol. 2006;20:150-2.
- Hawley WA, Reiter P, Copeland RS, et al. Aedes albopictus in North America: probable introduction in used tyres from northern Asia. Science. 1987;236:1114-6.