INTRODUCTION
Therapy for women who present with locally advanced squamous cell carcinoma cervix often fails to control loco regional disease because doses required to treat large tumor volumes exceed the limit of toxicity in normal tissue. To improve local control, a variety of innovate therapies have been evaluated including use of hyperthermia [1], hypoxic cell sensitizer [2], intra-arterial chemotherapy [3] and concurrent chemotherapy. Various drugs which has been used as a radio sensitizer for concurrent chemo radiation include hydroxyurea [4], mitomycin-C [5], 5-fluorouracil, Irinotecon, Topotecan, and Paclitaxel in combination with cisplatin [6-9]. Cisplatin based chemotherapy is now the standard of care for high risk or locally advanced cervical cancer [10].
Gemcitabine (2 deoxy 2'-2' diflurocytidine) is a novel deoxycytidine analogue, which was orginally investigated for its antiviral effect but has since been developed as an anticancer therapy. It is a cell-cycle specific (S- phase) cytotoxic agent that kills the cells in S-phase undergoing DNA synthesis. It also blocks cells through GI/S phase boundary. Recently it has been shown that Gemcitabine acts as a radio sensitizer in cervical cancer cell line [11]. Mc Cormach et al [12] used Gemcitabine with radiation in patients with locally advanced cervical cancer and concluded that Gemcitabine is more potent radio sensitizer than Cisplatin. Inspired by these results, the present study was conducted to compare the antitumor activity and toxicity of concurrent radiation with weekly Cisplatin or Gemcitabine in locally advanced cervical cancer.
MATERIALS AND METHODS
Women with untreated invasive squamous cell carcinoma of the cervix of FIGO (1994) stage IIB and IIIB were enrolled in this study from June 2001 to May 2002. All cases were confirmed histologically. Each patient was required to undergo a complete physical examination, a pelvic examination, chest radiography and intravenous pyelography (IVP) or abdominal computed tomography with intravenous contrast to determine the clinical stage of cancer.
Other eligibility criteria included Hb level >10mg/dl, WBC count > 4000/mm3, platelet count > 100 000/mm3 and serum creatinine < 1.2 gm/dl. Patients were randomized according to age, parity, FIGO stage, gross pathology and histopathology, and were divided into control arm (30 patients) and a trial arm (30 patients). In the control arm, patients received weekly Cisplatin 40mg/m2 intravenously x 5 cycles concurrent with EBRT 50Gy/25# as 5#/week. In the trial arm, patients received weekly Gemcitabine 100mg/m2 intravenously x 5 cycles concurrent with EBRT 50Gy/25# as 5#/week. All the patients received single application of intracavitory brachytherapy to deliver 20 Gy at point A, two weeks after completion of EBRT.
Prior to randomization, patients were informed about the treatment options and the risks of chemotherapy and radiotherapy. Response of treatment was recorded both subjectively and objectively in control arm and trial arm. Subjective response included greater than 50% relief in bleeding and discharges per vagina and pain abdomen. Objective response included complete clinical regression of tumor size six weeks after completion of therapy. Toxicity of treatment was graded according to WHO criteria.
RESULTS
Sixty patients were entered on study: 30 were assigned to receive radiotherapy and concomitant chemotherapy with Cisplatin; and 30 were assigned to receive radiotherapy and concomitant chemotherapy with Gemcitabine. There were no significant differences in the clinical characteristics among the two treatment group (Table 1).
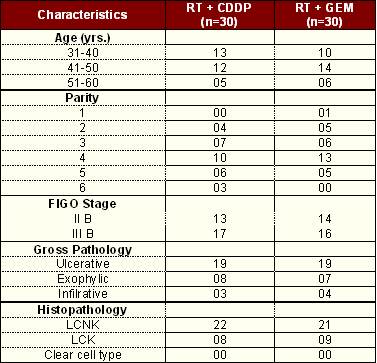 |
RT= Radiotherapy, CDDP = Cisplatin, GEM= Gemcitabine, n= No. of patients |
In this study both subjective and objective responses were better in the control arm i.e. in patients receiving Cisplatin concomitant with radiation. Subjective response was 29/30 (96.66%) vs. 24/30 (80%) and objective response was 28/30 (93.33%) vs. 21/30 (70%) (Table 2).
 |
CDDP = Cisplatin, RT = Radiotherapy, GEM = Gemcitabine |
Tumor regression was evaluated 6 weeks after completion of treatment. A statistically significant difference i.e. 93.33% vs. 70% (p=0.01) was noted between the two arm (Table 3).
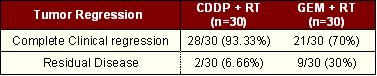 |
CDDP = Cisplatin, GEM = Gemcitabine, n= No. of patients |
Apart from nausea and vomiting, all toxicities were more frequent in patients receiving Gemcitabine + EBRT. Nausea and vomiting were more common in patients receiving Cisplatin + EBRT. There was no grade IV reaction in either arm. Grade III diarrhea was seen in patients receiving Gemcitabine + EBRT, but it was manageable. All patients in the control and trial arm received full course of treatment without any gap or interruption (Table 4).
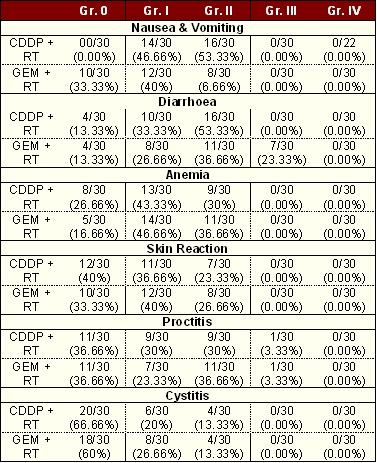 |
CDDP = Cisplatin, GEM = Gemcitabine, RT = Radiotherapy |
DISCUSSION
Radiation therapy had previously been the established treatment for locally advanced cervical cancer. Recently, five phase III trials have demonstrated a significant survival advantage for the concomitant administration of radiotherapy with Cisplatin based chemotherapy. Although the trials vary in terms of stages of disease, dose of radiation, and schedule of radiation and Cisplatin, they all demonstrated a significant survival benefit for the combined approach [13-17]. The National Cancer Institute of Canada (NCCI) trial however has shown no improvement in local control or overall survival with concomitant administration of Cisplatin to radiation [18]. Despite the result of this trial, Cisplatin based concomitant chemo-radiation is regarded as the standard treatment for locally advanced cervical cancer [10].
Gemcitabine is a cell-cycle specific cytotoxic agent that has shown antitumour activity against a variety of solid tumor e.g. lung, pancreas, breast and bladder. Recently Hernandez P et al [11] have demonstrated the radiosensitizing effect of Gemcitabine against cervical cancer cell lines. Pattaranutapern P et al [19] have also shown efficacy and feasibility of weekly concurrent Gemcitabine with radiation in stage IIIB cervical cancer.
In our study, the Cisplatin-based chemoradiation arm was found to be more effective and tolerable as compared to Gemcitabine-based chemoradiation arm. Similar results have been obtained in a recent study based on fifteen phase I/II clinical trials on the use of Gemcitabine, in cervical cancer, where Gemcitabine as a single agent was inferior to Cisplatin, when used concurrently with radiation [20].
Effectiveness of the treatment modality was judged not only by response, but also by the associated side effects. Nausea and vomiting were higher in patients receiving Cisplatin concomitant with radiation. Diarrhea, anemia and skin reactions were more common and severe in patients receiving Gemcitabine concomitant with radiation. There were no grade III reactions in the control arm but in the trial arm 7/30 (23.33%) patients developed grade III diarrhea.
CONCLUSION
Cisplatin is a better option than Gemcitabine when used as a radio sensitizer for locally advanced cervical cancer both in terms of response vs. toxicities. Gemcitabine as a single agent is less effective and feasible as compared to Cisplatin for use as a radio sensitizer for locally advanced cervical cancer. It remains to be shown in future trials whether the combination of both cisplatin and gemcitabine with concurrent radiation may prove to be superior to either single agent.
REFERENCES
-
Prosnitz L, Jones E. Test value of hyperthermia in patients with carcinoma of the cervix being treated with concurrent chemotherapy and radiation. Int. J. Hyperthermia 2002;18:3-8.
-
Stehman FB, Bundy BN, Thomas G, et al. Hydroxyurea vs. Misorudazole with radiation in cervical carcinoma: long term follow-up a gynaecologic oncology group trial. J. Clin. Oncol. 1993;11:1523-8.
-
Morris M, Eifel JP, Burke W.T. et al. Treatment of locally advanced cervical cancer with concurrent radiation and intra arterial chemotherapy. Gynaecol Oncol. 1995;57:72-78.
-
Hreshcy MM, Bernord AS, Borrow CR, et al. Hydroxyurea or placebo combined with radiation to treat stage IIIB and IV A cervical cancer confirmed to the pelvis. Int. J. Radiation Oncol. Biol. Phys. 1979;5:317-22.
-
Robert KB, Urdoneata N, Uera R, et al. Interim results of a randomized trial of mitomycuric as an adjunct to radical radiotherapy in the treatment of locally advanced squamous cell carcinoma of the cervix. Int. J. Cancer 2000;90:26-3.
-
Robert SW, Kavanages JJ, Greenberg H, et al. Concomitant radiation therapy and chemotherapy in the treatment of advanced squamous cell carcinoma of the lower female genital tract. Gynaecologic Oncology 1989;34:183-6.
-
Sugiyama T, Yakushiji M, Noda K, et al. Phase-II study of irinotecan and cisplatin as first line chemotherapy in advanced or recurrent cervical cancer. Oncology 2000;58:31-7.
-
Fiorica H, Holloway R, Ndubisi B, et al. trial of topotecan and cisplatin in persisted or recurrent sq. non sq. carcinoma of the cervix. Gynaecol. Oncol. 2002;85(1):89-94.
-
Pignata S, Frezzo P, Tramonto S, et al. Phase-I study with weekly cisplatin + Paclitaxel and concurrent radiotherapy in patients with carcinoma of the cervix uteri. 2000;11:455-9.
-
Vreololjak E, Hamm W. Current state-of-the-art of concomitant chemoradiation in carcinoma cervix. Eur. J. Gynaecol. Oncol. 2003;24(6):475-9.
-
Hernandez P, Oliverra P, Ducnas-Gonzalez A, et al. Gemcitabine activity in cervical cancer cell line. Cancer Chemotherapy Pharmacol. 2001;48:488-92.
-
McCormack M, Thomas H. A phase IB study of gematabine and concurrent radiotherapy in carcinoma of the cervix. Ann. of Oncol. 2000;11:88.
-
Keys HM, Bundy BN, Stehman FB, et al. Cisplatin; radiation adjuvant hysterectomy compared with radiation and adjuvant hysterectomy for bulky stage 1B cervical carcinoma. N. Eng. J. Med. 1999;340:1154-61.
-
Morris M. Eifel PJ, Lu J, et al. Pelvic radiation with concurrent chemotherapy compared with pelvic and para-aortic radiation for high risk cervical cancer. N. Engl. J. Med. 1999;340:1137-43.
-
Peters WAIII, Liu PY, Barrett RJ, et al. Cisplatin and SFU plus radiation therapy are superior to radiation therapy as adjunctive therapy in high risk early stage carcinoma of the cervix after radical hysterectomy and pelvic lymphadenectomy: report of a phase III intergroup study, presented at 30th annual meeting of the society of Gynaecologist, San Francisco CA March 1999;20-4.
-
Rose PG, Bundy BN, Watkins CB, et al. Concurrent cisplatin based radiotherapy and chemotherapy for locally advanced cervical cancer. N. Engl. J. Med. 1999;340:1144-53.
-
Whitney CW, Sauce W, Bundy BN, et al. A randomized comparison of fluorociracil plus cisplatin vs. hydroxyurea as an adjunct to radiation therapy in stage IIB - IV A carcinoma of the cervix with negative paraaortic lymphnode. A gynaecologic oncology group and South West Oncology group study. J. Clin. Oncol. 1999;17:1339-48.
-
Pearcey RG, Brundage MD, Drouin P, et al. A clinical trial comparing concurrent cisplatin and radiation therapy vs. radiation alone for locally advanced squamous cell carcinoma of the cervix carried out by the National Cancer Institute of Canada. Clinica Trial Group Proc. Ann. Soc. Clin. Oncol. 2000;19:3782.
-
Pattaranutapern P, Thirapakawong C, Chanshlka Y, et al. Phase II study of concurrent gemcitabine and radiotherapy in locally advanced stage IIIB Cervical carcinoma. Gynecologic Oncology 2001;81:404-7.
-
David G, Mutch MD, Bloss JD. Gemcitabine in cervical cancer. Gynecologic Oncology 2003;90:S8-S15.